Introduction to Good Experimental Technique:
- Water quality.
Ordinary tap water contains contaminants that can interfere with the reactions that take place within the experimental protocols. Purified water should ALWAYS be used to make up reagents. Distilled water is available from the faucet marked D.W. at each sink in the lab.
- Clean glassware.
Many contaminants adhere to the inner walls of glass containers. Glassware should be washed with dilute detergent followed by 5-10 water rinses. The final rinse should be with distilled water. 0.5% detergent (Liquinox) is available in squeeze bottles beside every sink in the lab.
- Solution prep.
Solids should be weighed on weighing paper or boats using an electronic analytical balance. These balances have been calibrated prior to the lab for accurate measurement.
Liquids are measured volumetrically using measuring cylinders, volumetric flasks and pipets. The hazardous properties of all materials should be known before use. Please refer to the MSDS and follow proper safety precautions.
- Solution storage
Reagent storage is critical for chemical stability and to reduce bacterial contamination. Containers must be sealed properly using stoppers, screw caps of parafilm. Reagents must also be stored at the appropriate temperature. Remember to clearly label containers with the contents and date prepared.
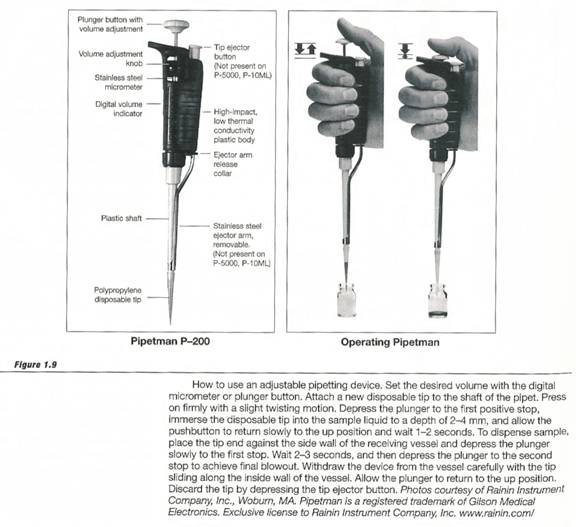
- Microliter pipettors
A microliter pipettor allows quick and accurate transfer of fixed volumes ranging from 1 to 10,000ml. In this lab three different sized pipettors will be used: p20, p200 and p1000. The number indicates the maximum number of microliters that can be transferred. They are adjustable, so that a p200 pipettor can transfer between 1 and 200mls. Although a p200 can dispense volumes less that 20ml, amounts in this range are more accurately measured using a p20 pipettor. Similarly the p1000 should be used to dispense volumes between 200ml and 1ml.
Correct pipettor operation and calibration are vital to obtaining accurate experimental results. The figure below illustrates correct pipettor operation. We will also calibrate the pipettors to be used in the lab.
- Sterile technique: working with sensitive biochemical’s
In the lab we will be working with unprotected nucleic acids and proteins. Since the environment, including human skin and airborne fungal spores are rich sources of proteases and nucleases; sterile technique is essential for handling these molecular biochemicals. Scientists should wear gloves, and use sterile pipet tips and containers where possible. Additionally, most molecular biochemicals are heat liable, and thus manipulations are often carried out on ice or even in a cold room.
7. Sterile technique: culturing cells.
All media, including plates, liquid media and top agar must be autoclaved immediately after they are prepared. It is best to prepare media in several small bottles, only opening one at a time. Use a flame on inoculating loops and on the lips of media bottles before and after pipetting from them. NOTE: We will be using disposable sterile plastic loops which should not be flame sterilized. Never leave a media or agar bottle open on the bench and don’t take an individually-wrapped pipet or loop out of its protective wrapper until you are ready to use it (i.e., don't walk across the room with an unwrapped pipet or loop). Always use a fresh, sterile pipet or pipet tip when pipetting culture media, and never go back into a media bottle or cell culture with a used pipet.
Ref: Biochemistry Laboratory, modern theory and techniques, Rodney Boyer.