CHEMISTRY HOME
BLACKBOARD LOGIN
LAB MANUAL HOME
SYLLABUS
| Spectroscopy is an analytical technique that scientists can use to determine the wavelength of light emitted or absorbed by different elements. So what, right? Well, spectroscopy can be very handy in that it allows us to determine the composition of the star or planet that is millions of miles away from the Earth by detecting those wavelengths of light emitted. We are able to do this because every element has its own unique set of emitted wavelengths of light. It is a like a fingerprint.
So what is the source of these unique wavelengths of light? As you have no doubt learned in class each atom contains electrons that reside at different energy levels around the nucleus. In order for an electron to change from one energy level to another, it must either release energy, which is called emission, or take in energy, which is called absorption. Because the levels of energy that surround the nucleus of each element are different, the amount of energy that is emitted or absorbed when moving between those levels will be individual to each element. The emission or absorption of energy is then related to the wavelength of light that you see by the relationship:
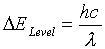
where DELevel is the energy that is being emitted or absorbed, h is a constant with a value of 6.626 x 10-34 J.s, c is the speed of light which is 3.00 x 108 m/s, and l (normally written in nm) is the scientific symbol used to represent a wavelength.
In today’s lab you are going to use the known wavelengths of emitted light for some elements to identify some unknowns. The table below lists the elements along with their wavelengths and corresponding colors. By measuring the wavelengths of light emitted from the unknown light source and comparing them to the known elemental wavelengths, you should be able to properly identify all of the unknowns.
Atom or Ion |
Wavelength (nm) |
Line Color |
Mercury |
405
436
546 |
Violet
Blue
Green-yellow |
Lithium |
671 |
Red |
Krypton |
435
588 |
Violet - Blue
Yellow |
Sodium |
589 |
Yellow-Orange |
|
|

