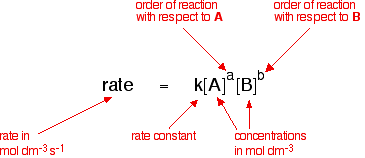
Rate constants and rate equations You will remember that the rate equation for a reaction between two substances A and B looks like this:

The rate equation shows the effect of changing the concentrations of the reactants on the rate of the reaction.
BUT, what about all the other things (like temperature and catalysts, for example) which also change rates of reaction? Where do these fit into this equation?
These are all included in the so-called rate constant (k)- which is only actually constant if all you are changing is the concentration of the reactants. If you change the temperature or the catalyst, for example, the rate constant changes.
This is shown mathematically in the Arrhenius equation.
The Arrhenius equation:
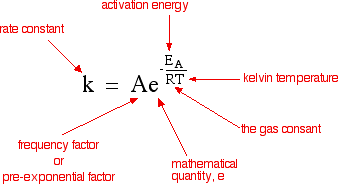
What the various symbols mean:
Temperature, T To fit into the equation, this has to be meaured in kelvin.
The gas constant, R is 8.31 J K-1 mol-1. This is a constant which comes from an equation, pV=nRT, which relates the pressure, volume and temperature of a particular number of moles of gas.
Activation energy, EA This is the minimum energy needed for the reaction to occur. To fit this into the equation, it has to be expressed in joules per mole. Below is a flash review of what Activation Energy is from (http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/activa2.swf).
The frequency factor, A You may also find this called the pre-exponential factor or the steric factor. A is a term which includes factors like the frequency of collisions and their orientation. It varies slightly with temperature, although not much. It is often taken as constant across small temperature ranges.
What are catalysts?
A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. When the reaction has finished, you would have exactly the same mass of catalyst as you had at the beginning.
Some common examples:
| reaction | catalyst |
|---|---|
| Decomposition of hydrogen peroxide | manganese(IV) oxide, MnO2 |
| Nitration of benzene | concentrated sulphuric acid |
| Manufacture of ammonia by the Haber Process | iron |
| Conversion of SO2 into SO3 during the Contact Process to make sulphuric acid | vanadium(V) oxide, V2O5 |
| Hydrogenation of a C=C double bond | nickel |
The key importance of activation energy:
Collisions only result in a reaction if the particles collide with enough energy to get the reaction started. This minimum energy required is called the activation energy for the reaction. A catalyst provides an alternative route for the reaction. That alternative route has a lower activation energy. Showing this on an energy profile:
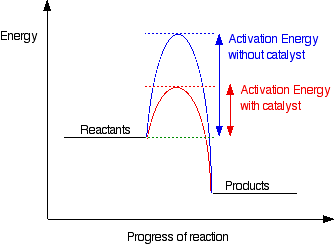
Be very careful if you are asked about this in an exam. The correct form of words is "A catalyst provides an alternative route for the reaction with a lower activation energy." It does not "lower the activation energy of the reaction".
There is a subtle difference between the two statements that is easily illustrated with a simple analogy. Suppose you have a mountain between two valleys so that the only way for people to get from one valley to the other is over the mountain. Only the most active people will manage to get from one valley to the other. Now suppose a tunnel is cut through the mountain. Many more people will now manage to get from one valley to the other by this easier route. You could say that the tunnel route has a lower activation energy than going over the mountain. But you haven't lowered the mountain! The tunnel has provided an alternative route but hasn't lowered the original one. The original mountain is still there, and some people will still choose to climb it.
In the chemistry case, if particles collide with enough energy they can still react in exactly the same way as if the catalyst wasn't there. It is simply that the majority of particles will react via the easier catalysed route.
Let's Practice:
Problems from http://science.widener.edu/svb/tutorial/arrheniuscsn7.html
And some more...
1) Consider the following reaction: 2 NOCl (g) --> 2 NO (g) + Cl2 (g) The rate constant for this reaction is 0.29 M-1 s-1 at 500 K and 16.3 M-1 s-1 at 600 K.
a. What is the slope of an Arrhenius plot for this reaction?
b. What is the activation energy for this reaction?
2) If the activation energy for a certain biological process is 50 kJ/mol (2 sig figs), by what factor will the rate of of this reaction increase when body temperature increases from 37°C (normal) to 41°C (fever)?
3) Given Ea for a certain biological process is 48 kJ/mol, and the rate constant is 2.5 x 10–2 s–1 at 15°C. What is the rate constant at 37°C?
4) Consider the following reaction: O + O3 => 2O2 The activation energy for this reaction is 25 kJ/mol and the enthalpy change, DH, is -388 kJ. What is the activation energy for the decomposition of O2 by the reverse reaction?