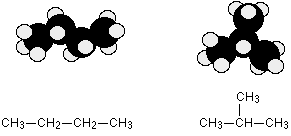
Hydrocarbons
Organic Molecules:
Organic chemistry is the study of the properties of the compounds of carbon. All carbon compounds except for a few inorganic carbon compounds are organic. Inorganic carbon compounds include the oxides of carbon, the bicarbonates and carbonates of metal ions, the metal cyanides, and a few others.
Hydrocarbons
The simplest Organic compounds are made up of only Carbon and Hydrogen atoms only. Compounds of Carbon and Hydrogen only are called Hydrocarbons.
Alkanes
The simplest Hydrocarbon is methane, CH4. This is the simplest member of a series of hydrocarbons. Each successive member of the series has one more Carbon atom than the preceding member. This series of compounds are called alkanes (CnH2n+2). The lighter ones are gases and used as fuels. The middle ones (7 Carbons to 12 Carbons) are liquids used in petrol (gasoline). The higher ones are waxy solids. Candle wax is a mixture of alkanes. Alkanes are saturated which means they contain the maximum number of hydrogens per carbon and no double or triple bonds.
The naming of organic compounds is referred to as organic nomenclature. There are a slew of rules for naming organic compounds that have been systemized by the International Union of Pure and Applied Chemistry
IUPAC Rules for Alkane Nomenclature
1. Find and name the longest continuous carbon chain.
2. Identify and name groups attached to this chain.
3. Number the chain consecutively, starting at the end nearest a substituent group.
4. Designate the location of each substituent group by an appropriate number and name.
5. Assemble the name, listing groups in alphabetical order.
The prefixes di, tri, tetra etc., used to designate several groups of the same kind, are not considered when alphabetizing.
Number of Carbons |
Prefix |
Structure |
1 |
Methane |
CH4 |
2 |
Ethane |
CH3CH3 |
3 |
Propane |
CH3CH2CH3 |
4 |
Butane |
CH3(CH2)2CH3 |
5 |
Pentane |
CH3(CH2)3CH3 |
6 |
Hexane |
CH3(CH2)4CH3 |
7 |
Heptane |
CH3(CH2)5CH3 |
8 |
Octane |
CH3(CH2)6CH3 |
9 |
Nonane |
CH3(CH2)7CH3 |
10 |
Decane |
CH3(CH2)8CH3 |
11 |
Undecane |
CH3(CH2)9CH3 |
12 |
Dodecane |
CH3(CH2)10CH3 |
Isomerism
All the alkanes with 4 or more carbon atoms in them show structural isomerism. This means that there are two or more different structural formulae that you can draw for each molecular formula.
For example, C4H10 could be either of these two different molecules:
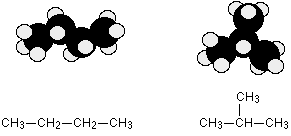
These are called respectively butane and 2-methylpropane.
Naming structural isomers of alkanes
The nomenclature becomes more complex if the alkane branches. In such a case, there are several rules that you must follow to give the alkane the correct name.
Applying the Rules
Now try applying these rules to name the following molecule (it's not as hard as it might seem).
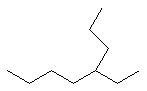
Take it step by step as outlined above.
1) Find the longest carbon chain in the molecule. First, begin by finding the parent chain in the molecule--that is, the longest possible chain of connecting carbons. Note that the parent chain is not necessarily the chain that simply follows from left to right. For example, if you were to count the number of carbons directly from left to right in this molecule you would get 7 carbons. This is not the parent chain, however! If you start at the left and then count up where the molecule branches, you find that there are 8 carbons by taking this route. This is is the longest chain (dont be fooled by professors hiding carbons in branches), and thus the parent chain is octane (see table above).
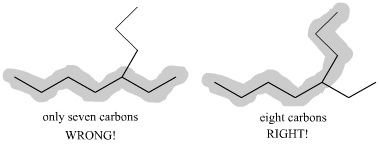
2) Number the parent chain. The second step is to number the carbons in the parent chain starting at the end closest to the first substituent. It is important to number the molecule from the correct end (in other words, in this example do you number the alkane from right to left or left to right). Following this rule, on this molecule you number from right to left, as the 2-carbon substituent is closer to that end.
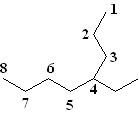 |
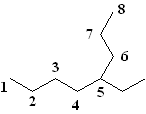 |
| Correct Numbering | Incorrect Numbering |
3. Name all the substituents. You then identify the names of the substituents. In this case, the only substituent is a 2 carbon group at the number 4 carbon. This is an ethyl group.
4. Put the substituents in alphabetical order. The next step is to put the substituents in alphabetical order (ie. ethyl before methyl) but since there is only one substituent this is unnecessary.
5. Locate the substituent on the parent change by giving it a number. Thus, the proper nomenclature of this alkane is 4-ethyloctane. Note that a dash is used to separate the number from the substituent.
Cycloalkanes again only contain carbon-hydrogen bonds and carbon-carbon single bonds, but this time the carbon atoms are joined up in a ring. The smallest cycloalkane is cyclopropane.
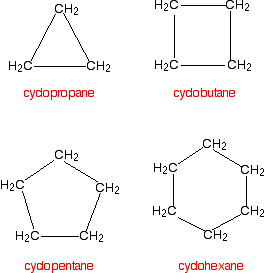
If you count the carbons and hydrogens, you will see that they no longer fit the general formula CnH2n+2. By joining the carbon atoms in a ring, you have had to lose two hydrogen atoms.
You are unlikely to ever need it, but the general formula for a cycloalkane is CnH2n.
Don't imagine that these are all flat molecules. All the cycloalkanes from cyclopentane upwards exist as "puckered rings".
Cyclohexane, for example, has a ring structure which looks like this:
This is known as the "chair" form of cyclohexane - from its shape which vaguely resembles a chair.
IUPAC Rules for Cycloalkane Nomenclature
1. For a monosubstituted cycloalkane the ring supplies the root name and the substituent group is named as usual. A location number is unnecessary.
2. If the alkyl sustituent is large and/or complex, the ring may be named as a substituent group on an alkane.
3. If two different substituents are present on the ring, they are listed in alphabetical order, and the first cited substituent is assigned to carbon #1. The numbering of ring carbons then continues in a direction (clockwise or counter-clockwise) that affords the second substituent the lower possible location number.
4. If several substituents are present on the ring, they are listed in alphabetical order. Location numbers are assigned to the substituents so that one of them is at carbon #1 and the other locations have the lowest possible numbers, counting in either a clockwise or counter-clockwise direction.
5. The name is assembled, listing groups in alphabetical order and giving each group (if there are two or more) a location number. The prefixes di, tri, tetra etc., used to designate several groups of the same kind, are not considered when alphabetizing.
Alkenes
Another series of compounds is called the alkenes. These have a general formula: CnH2n. Alkenes have fewer hydrogen atoms than the alkanes. The extra valencies left over occur as double bonds between a pair of Carbon atoms. The double bonds are more reactive than single bonds making the alkenes chemically more reactive.
IUPAC Rules for Alkene and Cycloalkene Nomenclature
1. The ene suffix (ending) indicates an alkene or cycloalkene.
2. The longest chain chosen for the root name must include both carbon atoms of the double bond.
3. The root chain must be numbered from the end nearest a double bond carbon atom. If the double bond is in the center of the chain, the nearest substituent rule is used to determine the end where numbering starts.
4. The smaller of the two numbers designating the carbon atoms of the double bond is used as the double bond locator. If more than one double bond is present the compound is named as a diene, triene or equivalent prefix indicating the number of double bonds, and each double bond is assigned a locator number.
5. In cycloalkenes the double bond carbons are assigned ring locations #1 and #2. Which of the two is #1 may be determined by the nearest substituent rule.
6. Substituent groups containing double bonds are:
H2C=CH– Vinyl group
H2C=CH–CH2– Allyl group
Alkynes
A third series are the alkynes. These have the following formula: CnH2n-2.
Alkynes have two carbon atoms joined by a triple bond. This is highly reactive making these compounds unstable.
IUPAC Rules for Alkyne Nomenclature
1. The yne suffix (ending) indicates an alkyne or cycloalkyne.
2. The longest chain chosen for the root name must include both carbon atoms of the triple bond.
3. The root chain must be numbered from the end nearest a triple bond carbon atom. If the triple bond is in the center of the chain, the nearest substituent rule is used to determine the end where numbering starts.
4. The smaller of the two numbers designating the carbon atoms of the triple bond is used as the triple bond locator.
5. If several multiple bonds are present, each must be assigned a locator number. Double bonds precede triple bonds in the IUPAC name, but the chain is numbered from the end nearest a multiple bond, regardless of its nature.
6. Because the triple bond is linear, it can only be accommodated in rings larger than ten carbons. In simple cycloalkynes the triple bond carbons are assigned ring locations #1 and #2. Which of the two is #1 may be determined by the nearest substituent rule.
7. Substituent groups containing triple bonds are:
HC≡C– Ethynyl group
HC≡CH–CH2– Propargyl group
Problems:
Draw the Structural Formula for 2-bromo-4,4-dichloro-5,5-dimethylheptane
Draw the Structural Formula for 4-bromo-1-ethylcyclopentene
Draw the Structural Formula for 7-methyl-6-octen-1,3-diyne