Practice Problems Answers:
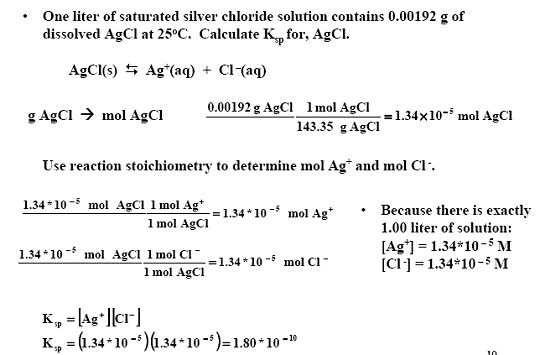
1) One liter of saturated silver chloride solution contains 0.00192 g of dissolved AgCl at 25oC. Calculate Ksp for, AgCl.

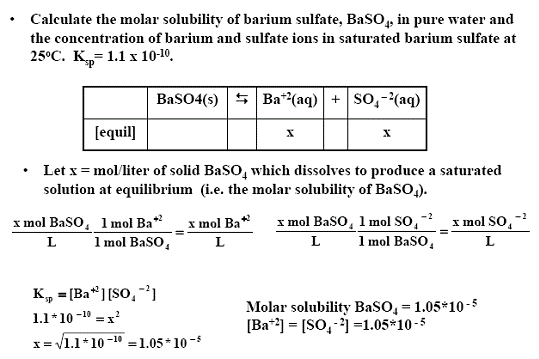
2) Calculate the molar solubility of barium sulfate, BaSO4, in pure water and the concentration of barium and sulfate ions in saturated barium sulfate at 25oC.
Ksp= 1.1 x 10-10.
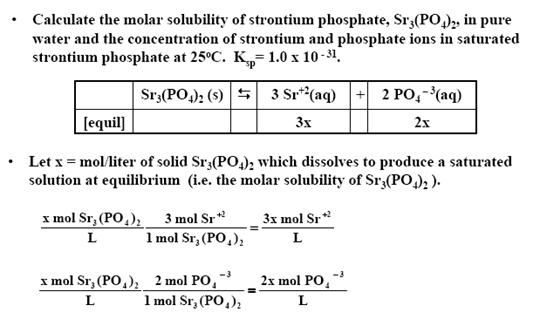
3) Calculate the molar solubility of strontium phosphate, Sr3(PO4)2, in pure water and the concentration of strontium and phosphate ions in saturated strontium phosphate at 25oC. Ksp= 1.0 x 10- 31.