

What is a Solution?
Solutions are homogeneous mixtures of two or more pure substances. For our purposes, we will generally be discussing solutions containing a single solute and water as the solvent. What is a solvent? In crudest terms it is the molecule in the mixture with the highest concentration. That is to say if you had a liter of salt and 2 grams of water. In that case, the salt would be the solvent and the water the solute. But this type of mixture would be useless so why bother to make it???
When we do place solutes and solvents together, there is what we call the solution process. You can think of it as being similar to what you would experience if you tried to squeeze into an already packed elevator. Everyone has to adjust to "find their space" again. Now just like in the elevator, molecules will adjust differently dependent on the type of molecule making an entrance. And also like in an elevator there will come a point when no more people can be added. For a solution, this point is called the saturation point and the solution itself is called a saturated solution. At the point of saturation, no more solute will dissolve in the solvent. Rather the process of dissolving and precipitation are both occurring simultaneously and at the same rate.


Generally speaking only certain molecules will dissolve in water to begin with. The old phrase "like dissolves like" or "birds of a feather flock together" is very true with respect to what degree solutes are soluble or miscible in different solvents. At very low concentrations, almost all molecules are somewhat soluble in all solvents. But by trend, ionic and polar solutes are more soluble in polar solvents and non-polar molecules are soluble in non-polar (mostly organic) solvents. The units of concentration we just discussed are used to describe the degree to which a solute is soluble in a solvent.
When you place a non-polar molecule in a polar solvent (like oil in water) the molecules try to minimize surface contact between them. (like you and a guy with a cold on an elevator). This is actually the basis for the cells in our bodies. The lipids (oily fatty acids) form our cell membranes so that their non-polar tails face inward away from the polar cytoplasm and the polar heads face towards the polar cytoplasm.
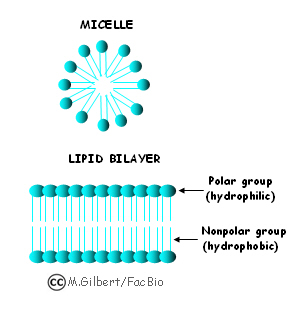
Why Do Solutions Form?
Although much of the explanation for why certain substances mix and form solutions and why others do not is beyond the scope of this class, we can get a glimpse at why solutions form by taking a look at the process by which ethanol, C2H5OH, dissolves in water. Ethanol is actually miscible in water, which means that the two liquids can be mixed in any proportion without any limit to their solubility. Much of what we now know about the tendency of particles to become more dispersed can be used to understand this kind of change as well.
Picture a layer of ethanol being carefully added to the top of some water (Figure below). Because the particles of a liquid are moving constantly, some of the ethanol particles at the boundary between the two liquids will immediately move into the water, and some of the water molecules will move into the ethanol. In this process, water-water and ethanol-ethanol attractions are broken and ethanol-water attractions are formed. Because both the ethanol and the water are molecular substances with O−H bonds, the attractions broken between water molecules and the attractions broken between ethanol molecules are hydrogen bonds. The attractions that form between the ethanol and water molecules are also hydrogen bonds (Figure below).
Because the attractions between the particles are so similar, the freedom of movement of the ethanol molecules in the water solution is about the same as their freedom of movement in the pure ethanol. The same can be said for the water. Because of this freedom of movement, both liquids will spread out to fill the total volume of the combined liquids. In this way, they will shift to the most probable, most dispersed state available, the state of being completely mixed. There are many more possible arrangements for this system when the ethanol and water molecules are dispersed throughout a solution than when they are restricted to separate layers. (Figure below).
We can now explain why automobile radiator coolants dissolve in water. The coolants typically contain either ethylene glycol or propylene glycol, which, like ethanol and water, contain hydrogen-bonding O−H bonds.
These substances mix easily with water for the same reason that ethanol mixes easily with water. The attractions broken on mixing are hydrogen bonds, and the attractions formed are also hydrogen bonds. There is no reason why the particles of each liquid cannot move somewhat freely from one liquid to another, and so they shift toward the most probable (most dispersed), mixed state.
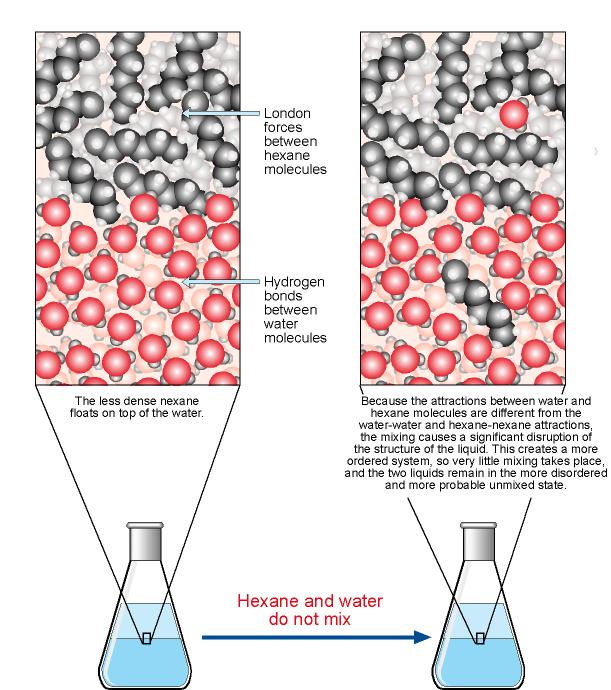
We have a different situation when we try to mix hexane, C6H14, and water. If we add hexane to water, the hexane will float on the top of the water with no apparent mixing. The reasons why hexane and water do not mix are complex, but the following gives you a glimpse at why hexane is insoluble in water.
There actually is a very slight mixing of hexane and water molecules. The natural tendency toward dispersal does lead some hexane molecules to move into the water and some water molecules to move into the hexane. When a hexane molecule moves into the water, London forces between hexane molecules and hydrogen bonds between water molecules are broken. New attractions between hexane and water molecules do form, but because the new attractions are very different from the attractions that are broken, they introduce significant changes in the structure of the water. It is believed that the water molecules adjust to compensate for the loss of some hydrogen bonds and the formation of the weaker hexane-water attractions by forming new hydrogen bonds and acquiring a new arrangement.
Overall, the attractions in the system after hexane and other hydrocarbon molecules move into the water are approximately equivalent in strength to the attractions in the separate substances. For this reason, little energy is absorbed or evolved when a small amount of a hydrocarbon is dissolved in water. To explain why only very small amounts of hydrocarbons such as hexane dissolve in water, therefore, we must look at the change in the entropy of the system. It is not obvious, but when hexane molecules move into the water layer, the particles in the new arrangement created are actually less dispersed (lower entropy) than the separate liquids. The natural tendency toward greater dispersal favors the separate hexane and water and keeps them from mixing.
This helps explain why gasoline and water do not mix. Gasoline is a mixture of hydrocarbons, including hexane. Gasoline and water do not mix because the nonpolar hydrocarbon molecules would disrupt the water in such a way as to produce a structure that was actually lower entropy; therefore, the mixture is less likely to exist than the separate liquids.
We can apply what we know about the mixing of ethanol and water to the mixing of two hydrocarbons, such as hexane, C6H14, and pentane, C5H12. When the nonpolar pentane molecules move into the nonpolar hexane, London forces are disrupted between the hexane molecules, but new London forces are formed between hexane and pentane molecules. Because the molecules are so similar, the structure of the solution and the strengths of the attractions between the particles are very similar to the structure and attractions found in the separate liquids. When these properties are not significantly different in the solution than in the separate liquids, we can assume that the solution has higher entropy than the separate liquids. Therefore, when very similar liquids, like pentane and hexane, are mixed, the natural tendency toward increasing entropy drives them into solution.
Exothermic changes lead to an increase in the energy of the surroundings, which leads to an increase in the number of ways that that energy can be arranged in the surroundings, and therefore, leads to an increase in the entropy of the surroundings. Endothermic changes lead to a decrease in the energy of the surroundings, which leads to a decrease in the number of ways that that energy can be arranged in the surroundings, and therefore, leads to a decrease in the entropy of the surroundings. Therefore, exothermic changes are more likely to occur than endothermic changes. We can use this generalization to help us explain why ionic compounds are insoluble in hexane. For an ionic compound to dissolve in hexane, ionic bonds and attractions between hexane molecules would need to be broken, and ion-hexane attractions would form. The new attractions formed between the ions and hexane would be considerably weaker than the attractions broken, making the solution process significantly endothermic. The tendency to shift to the higher entropy solution cannot overcome the decrease in the entropy of the surroundings that accompanies the endothermic change, so ionic compounds are insoluble in hexane.
Ionic compounds are often soluble in water, because the attractions formed between ions and water are frequently strong enough to make their solution either exothermic or only slightly endothermic. For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. Even if the solution is slightly endothermic, the tendency to shift to the higher entropy solution often makes ionic compounds soluble in water.
The dividing line between what we call soluble and what we call insoluble is arbitrary, but the following are common criteria for describing substances as insoluble, soluble, or moderately soluble.
If less than 1 gram of the substance will dissolve in 100 milliliters (or 100 g) of solvent, the substance is considered insoluble.
If more than 10 grams of substance will dissolve in 100 milliliters (or 100 g) of solvent, the substance is considered soluble.
If between 1 and 10 grams of a substance will dissolve in 100 milliliters (or 100 g) of solvent, the substance is considered moderately soluble.
Although it is difficult to determine specific solubilities without either finding them by experiment or referring to a table of solubilities, we do have guidelines that allow us to predict relative solubilities. Principal among these is
Like dissolves like.
For example, this guideline could be used to predict that ethanol, which is composed of polar molecules, would be soluble in water, which is also composed of polar molecules. Likewise, pentane (C5H12), which has nonpolar molecules, is miscible with hexane, which also has nonpolar molecules. We will use the Like Dissolve Like guideline to predict whether a substance is likely to be more soluble in water or in hexane. It can also be used to predict which of two substances is likely to be more soluble in water and which of two substances is likely to be more soluble in a nonpolar solvent, such as hexane:
Polar substances are likely to dissolve in polar solvents. For example, ionic compounds, which are very polar, are often soluble in the polar solvent water.
Nonpolar substances are likely to dissolve in nonpolar solvents. For example, nonpolar molecular substances are likely to dissolve in hexane, a common nonpolar solvent.
Two additional guidelines are derived from these:
Nonpolar substances are not likely to dissolve to a significant degree in polar solvents. For example, nonpolar molecular substances, like hydrocarbons, are likely to be insoluble in water.
Polar substances are not likely to dissolve to a significant degree in nonpolar solvents. For example, ionic compounds are insoluble in hexane.
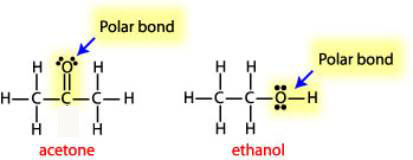
It is more difficult to predict the solubility of polar molecular substances than to predict the solubility of ionic compounds and nonpolar molecular substances. Many polar molecular substances are soluble in both water and hexane. For example, ethanol is miscible with both water and hexane. The following generalization is helpful:
Substances composed of small polar molecules, such as acetone and ethanol, are usually soluble in water. (They are also often soluble in hexane.)
Type of Substance Soluble in Water? Soluble in Hexane? Ionic Compounds Often No Molecular Compounds with Nonpolar Molecules No Yes Molecular Compounds with Small Polar Molecules Usually Often
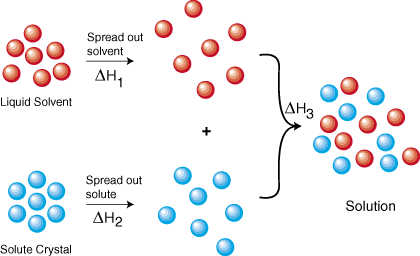
The process of dissolving is a process which involves the breaking and making of bonds, and that involves energy. From Hess's law we know that we can add the energies of each step in the cycle to determine the energy of the overall process. Therefore, the energy of solution formation, the enthalpy of solution, equals the sum of the three steps: DHsoln = DH1 + DH2 + DH3.
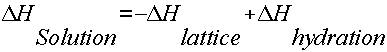
Click anywhere on the graphic below to start the animation. NOTE: You will need Java Script to run it.