|
Throughout this semester we have tried to provide experiments that illustrate some of the more complex concepts of chemistry as you were learning about them in lecture. Additionally, we have also tried to present you with evidence that each of the concepts or techniques that you have covered in this lab were either valuable to your future as a chemist or relevant to your everyday life. This experiment on Gas Laws and Airbags is both. Gas Laws and the general concepts of gas volumes and pressures are vital for anyone deciding on a career in chemistry and even more important to those focussing on medicine. For example, oxygen saturation in the body is controlled by pressures both internal and external. Why is it harder to breathe at higher altitudes? What is hypoxia? What happens to a patient if they receive too much oxygen? Not enough? And how do you as the doctor or nurse control the volume delivered? None of these questions can be answered without a firm understanding of the Gas Laws. The experiment you will be conducting today will not require you to administer oxygen to a patient (hopefully) but rather focusses on another very useful device that is equally dependent on the use of Gas Laws, the automoble airbag. Airbag deployment is chemically rather than mechanically created. A mechanical deployment would be too slow. An explosive creation of nitrogen gas from sodium azide creates the filled airbag in a fraction of a second. Gas Laws allow the construction of a completely full but not overfull bag of gas that keeps the driver and passenger(s) of a car from serious injury during a collision. In order to study how Gas Laws can provide answers to the construction of airbags, you are going to design and build one in lab. In addition to the construction of the airbag, we are also going to revisit the use of a barometer to measure the atmospheric pressure.
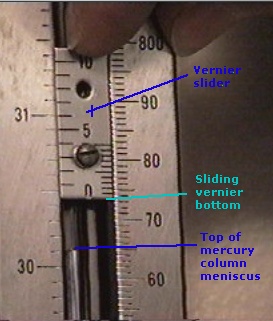
The barometer in the lab uses a vernier scale, so make sure you remember how to read this type of scale.
|
 |
Gas Laws and Airbags