|
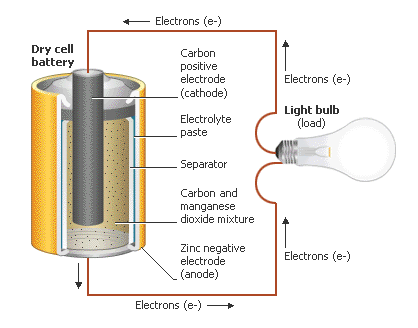
Have you ever wondered how a battery worked? We use them everyday for our cell phones, our cars, our mp3 players and even some important things like pacemakers. But have you ever really thought about how or why they work? What was inside of them? For one second, just imagine that there was no power, no electricity coming from the plug in the wall, but you desperately needed power to make something work and your life depended on it. What would you do? What (as a chemist) could you do? Electrochemistry is the study of electrochemical reactions. These are reactions that create a flow of electrons from one place to another. A flow of electrons is by definition "electricity". A battery is a small holding cell of electrochemical energy. In each battery there are two reserviors of chemicals that if connected by a conductive material will commence a transfer of electrons. To use this flow of electrons (electricity) to power our devices we simply need to wire them into the loop:
As a chemist, you should know the basic design of a common battery and also the chemistry behind why it works. In this lab, we are going to create some small voltaic cells out of various chemicals and measure their potentials (e.g. how strong the flow of electrons is from one metal to another). The stronger the electron flow from one metal to the other, the greater the potential difference between the two metals and the more electricity that can be garnered. A lot of research has been devoted to the production of a better battery. The lithium batteries that power our laptops are the result of some of that research, being approximately twice as strong as a normal zinc-carbon battery. Development of a potential series of metals therefore is a valuable tool for a chemist, allowing him or her to select metals/chemicals that will produce the most power. Click here to see a table of potentials. In addition to learning about redox reactions, potentials and the chemistry behind a battery, you will also be learning to use a new device called a multimeter:
We will use the multimeter in the lab to measure the potentials of the cells that we construct. A website of special interest. Check it out: Build a Lemon Battery
|
 |
Redox Reactions in Voltaic Cells: Construction of a Potential Series