Have you ever wondered how scientists know what elements are present on other planets?
As shown above, each of these heavenly bodies has an emission spectrum that can be read from far away using a device called a spectrometer ( ..and a really powerful telescope too.) But the spectrometer is able to separate the colors of the light being emitted by the star etc. into discrete lines. The origin of these lines is discussed further in both your class text and also in the Background section for this lab. But generally each element has a set of these lines individual to itself and thus if you have a sophisticated computer program that can separate the individual spectra from the mass of lines seen above, you can tell what elements are present in galaxies far, far away... In today's lab we will be working a little closer to home to practice both the use of our spectroscopes and to learn to interpret the meaning of a spectrum. You will be experimentally determining the spectra of several unknowns and then using known "bright line" spectra (see Background) for comparison, identifying the unknown ions.
|
 |
Atomic Spectra
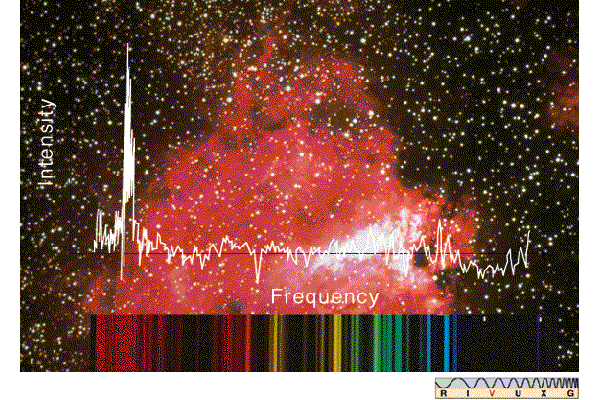 Visible Spectrum Of M17 - The visible spectrum of the hot gases in a nearby star-forming region known as the Omega nebula (M17). Shining by the light of several very hot stars, the nebula produces a complex spectrum of bright and dark lines (bottom), also shown here as an intensity trace from red to blue (center).
Visible Spectrum Of M17 - The visible spectrum of the hot gases in a nearby star-forming region known as the Omega nebula (M17). Shining by the light of several very hot stars, the nebula produces a complex spectrum of bright and dark lines (bottom), also shown here as an intensity trace from red to blue (center). 