 Research at the Alabugin Group:
Research at the Alabugin Group: Making new C-C and C-H bonds in cascade transformations of alkynesWe take advantage of the synthetic opportunities offered by the simultaneous presence of two reactive p-bonds of the alkyne moiety for the design of new cascade transformations of alkynes. In these reactions, one can form up to four new C-C bonds, both single and double, and/or couple the C-C bond formation with the C-H bond formation. In the exteme cases, one can move the cascade even further and break all three bonds between the two alkyne carbons, creating six new bonds! 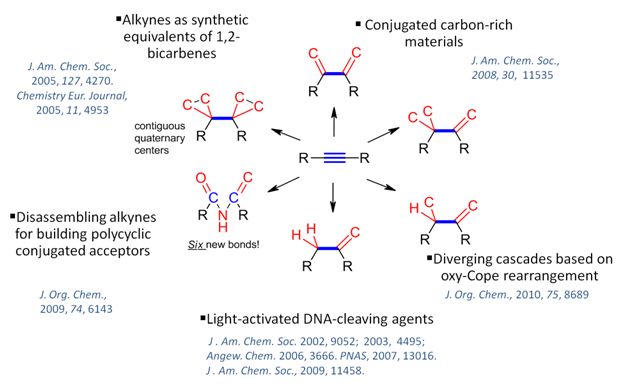 Mixing C-H and C-C bonds formation from alkynes: |
Building carbon nanostructures from scratch?We found a remarkable reaction which transform a tris-alkyne into a larger polycyclic structure via a radical five-step cascade (with ~93% yield per step). DFT calculations predict the key reactions in this sequence (5-exo-dig and 6-exo-dig cyclizations) to have very low barriers. Encouraged by these results, we are redesigning this cascade transformation towards preparation of larger carbon-nanostructures related to graphene nanoribbons and tips of carbon nanotubes.
The parachute reaction: alkynes as synthetic equivalents of 1,2-bicarbenesIn a very interesting photochemical "cycloaddition", alkyne form four new C-C bonds and two contiguous stereocenters. From a topological perspective, alkyne behave in this reaction as synthetic equivalents of 1,2-bicarbenes. However, the mechanism is more complex and interesting. We suggested that it involves a "boomerang" radical addition/cyclization sequence (read more..) and used this reaction for the preparation of an interesitng family of porous hybrid organic/inorganic materials (read more..). There are indications that this reaction will help us to understand the mechanism of photochemical DNA-modification by alkynes. We are also working on expanding the scope of this process and develop it into a photochemical analog of "click chemistry". 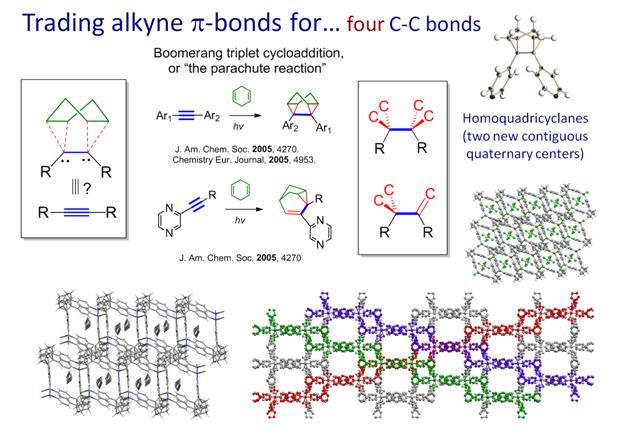 |