 Research at the Alabugin Group:
Research at the Alabugin Group: DNA as a target
Selectivity and efficiency are the most important questions in the design of DNA-cleaving agents for cancer therapy. How can one limit the damage to the cancerous cells without affecting the healthy tissue? How can one overpower the efficient DNA repair mechanisms which usually reverse the damage and keep our genome functioning for many years? The astounding biological activity of natural enediyne antibiotics is associated with their ability to cause simultaneous damage of both DNA strands ("the doble stranded" or "ds" DNA cleavage). Although even the best of natural enediynes, calicheamicin, induces, at best, ~25-33% of the double stranded cleavage, this is still enough to account for the record-breaking biological activity of enediynes (and also set a world record for ds:ss (single-strand) DNA cleavage ratio for a non-enzymatic molecule).
Our group developed a family of light-activated molecules with the ds-DNA cleavage efficiency rivaling (for the first generation) or exceeding (for the second generation) the ds:ss ratio of calichiamicin. Not only the use of light allows us to activate our molecules exactly at the right time and right place, inside of the cancer cells but, remarkably, the unprecedently high efficiency of ds-DNA cleavage is observed under slighly acidic conditions suitable for selective targeting of certain cancers. Photochemical conversion of ss DNA damage to ds DNA damageWe have developed the first light-activated molecular system which can convert easily repairable ss DNA cleavage into ds DNA cleavage (read more: the original work, press-release, media coverage...)
|
pH-Gated light-activated reagents for ds-DNA cleavageIn our design, we combined efficient DNA-cleaver capable of operating within the physiological pH range with a pH-controlled part which allows us to "switch on" the reactivity at a relatively narrow and predefined pH point close to the threshold between cancerous and healthy cells (read more..). 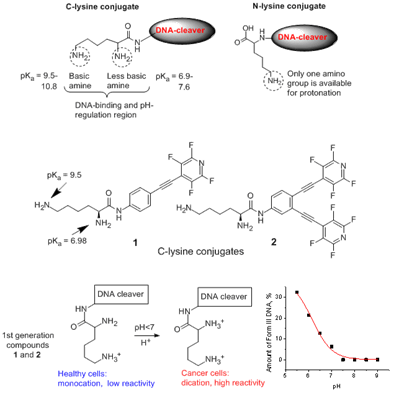 At pH 5.5, we observe up to 50% of ds DNA cleavage. Remarkably, at the same concentrations and pH of healthy cells (pH 7.2), almost no double stranded damage is observed! 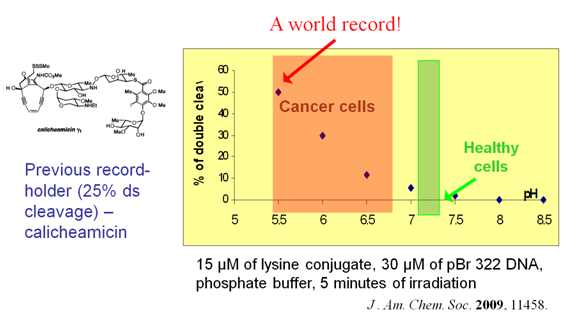 |