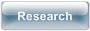
Esperamicin fragmentations
Biological activity of natural enediyne antibiotics stems from the formation of 1,4-dehydrobenzene diradical via Bergman cycloaromatization of the enediyne functional core. The diradical can abstract hydrogen atoms from the two strands of the DNA duplex, causing double strand DNA cleavage and subsequent apoptosis, i.e. self-programmed cell.
As a result of this remarkably efficient process, enediynes display activity against a wide range of human tumor cell lines at very low concentrations. For example, C1027 displays cytotoxicity against KB carcinoma cells with IC50 of 0.1 ng/ml in vitro. It is astonishing that molecules with such levels of toxicity can be produced and utilized by microorganisms without sustaining lethal self-damage
Many of the natural enediynes, like esperamicins and calicheamicins have a carbohydrate moiety positioned directly next to the enediyne functionality. It is generally established that the carbohydrate part plays an important role of binding to DNA and orienting the enediyne warhead appropriately relative to the target. However, other functions of the carbohydrate moiety are less understood. For example, the sugar part of esperamicin A1 fragments from the aromatic "core" upon cycloaromatization. We have prepared molecules which mimic the carbohydrate moiety of esperamicins and investigated radical fragmentations in these substrates in detail.
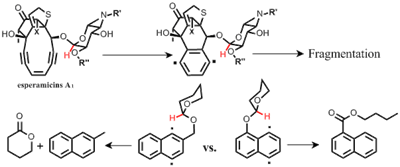
Our observations combined with the available literature data, fit well into the following logical progression of facts and observations: a) Esperamicins are produced by microorganisms and exported outside of the cell in a full, unfragmented form; b) Esperamicins fragment into the aromatized and carbohydrate parts upon their activation and subsequent Bergman cyclization; c) compounds, designed to mimic esperamicins, also undergo fragmentation after their Bergman cyclization; d) chemical similarity between our molecules and esperamicins suggests that these fragmentations proceed via a similar mechanism; and e) this “conformationally gated” mechanism depends on the orientation of the carbohydrate moiety because its proximity to the p-benzyne radical is required to enable the intramolecular H-abstraction step. We combined these observations in an intriguing hypothesis on how microorgamisms producing these lethal toxins may protect themselves from their own poison. Read more»
New Radical Reactions: Metal-free conversion of phenols to benzoates and benzamides
We designed a new radical fragmentation which can serve as a synthetic shortcut between phenols and benzoates. 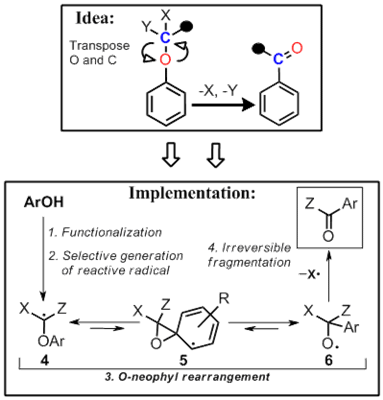
The successful design included several factors. First, phenols should be converted readily to a functional precursor of a phenoxy-substituted carbon radical. Second, this radical should initiate the key 1,2 O→C transposition on the aromatic ring through an O-neophyl rearrangement. Finally, since the O-neophyl rearrangement is reversible, a fast and selective fragmentation via efficient β-scission of a weak C─X bond should be available in order to trap the transposed radical.