The Missing C1-C5 Cycloaromatization Reaction
"A policeman sees a drunk man searching for something under a streetlight and asks what the drunk has lost. He says he lost his keys and they both look under the streetlight together. After a few minutes the policeman asks if he is sure he lost them here, and the drunk replies, no, and that he lost them in the park. The policeman asks why he is searching here, and the drunk replies, "this is where the light is."" (https://en.wikipedia.org/wiki/Streetlight_effect)
Chemists from Florida State University had shown that the drunkard was correct - just shining light can help to find things that are hard to find otherwise. Because, in chemistry, light itself can create things/processes/reactions that would never even exist in the dark.
The 2015 year is the International Year of Light. Below is an illustration of how unique properties of light-activated reactions allowed the discovery of the last one from the four archetypal cycloaromatization reactions.
Cycloaromatization reactions defy common chemical logic by creating diradical species from closed shell reactants without external radical initiators. The four ways in which acyclic reactants such as enediynes and enynes can transform into 1,4-diradicals is shown in Figure 1. In these unusual but very useful processes, one chemical bond is always created at the expense of two chemical bonds that are sacrificed.
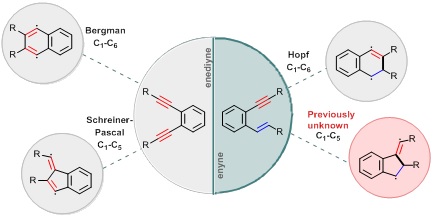
Figure 1. The possible cycloaromatization modes for enediynes (left) and enynes (right).
The explosion of interest in cycloaromatization reactions dates back to the discovery of natural enediyne antibiotics, the closed-shell molecules that are transformed by the Bergman cycloaromatization into reactive diradical species capable of targeting and damaging cellular DNA with astounding efficiency via rapid and irreversible H atom abstraction from the sugar phosphate backbone. Natural enediynes, such as Esperamicin A, Calicheamicin λ 1, and Dynemicin A, are hailed as the most potent family of anticancer agents discovered to date (Figure 2). The simultaneous formation of two radical centers is important because it allows abstraction of two hydrogen atoms, enabling double-strand DNA cleavage.
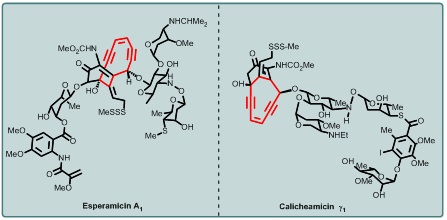
Figure 2. Natural enediynes, such as Esperamicin A, Calicheamicin λ 1 are potent anticancer agents.
Considering the broad interest in cycloaromatization reactions, it is remarkable that the last member of the cycloaromatization reaction family, an efficient C1-C5 cyclization of enynes, had remained unknown. Now, researchers at Florida State University have employed an innovative solution in order to overcome the possible problems associated with trapping the diradical product of the thus far elusive process (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b07448 ). By utilizing a "self-terminating" reaction strategy, based on previous methodology the group has developed, the cyclization is terminated by a stereoelectronically promoted and thermodynamically favorable carbon-carbon bond fragmentation.
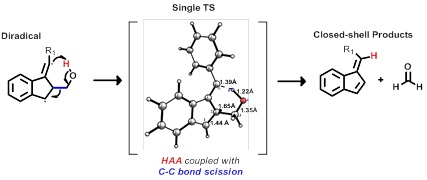
In this light promoted reaction cascade, the C-C fragmentation is coupled with an intramolecular H-abstraction made possible by the introduction of a pendant CH2OH moiety in an unprecedented reaction that converts the diradical directly into a closed shell species via a single concerted step, Figure 3. Such transformation allows facile intramolecular trapping of unstable radicals that are formed transiently and have a short lifetime.
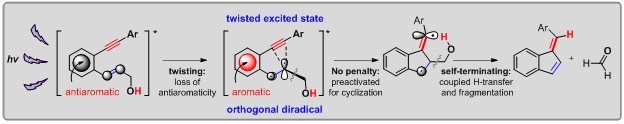
Figure 3. The sequence of steps for inducing and trapping the C1-C5 closure of enynes, the last missing member of the cycloaromatization family.
With the discovery of this efficient photochemical incarnation of one of the four basic archetypical diyne and enyne cyclizations illustrates the potential of excited state antiaromaticity alleviation to initiate and control photochemical reaction, in addition to providing a procedure for the photo-release of formaldehyde. The applications of this chemistry are not limited; the utility of enediynes and enynes in biology, organic synthesis and in the preparation of advanced polymers and carbon-rich materials continues to evolve, fueled by innovative and unique approaches.