Polymer and Materials Science
About us
Innovative concepts are the driving force behind our research. We use polyelectrolytes to make new materials and to stimulate new responses at the biological interface. Our methods focus on practical ways to coax extraordinary behavior from familiar materials.
Browse our publicationsSaloplastics
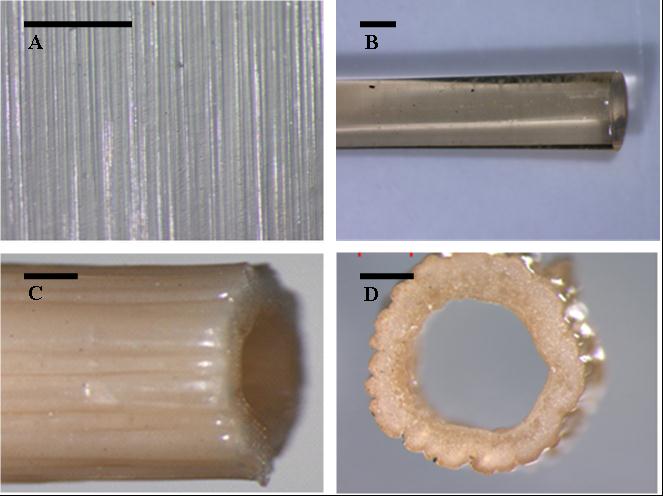
Imagine melting polymers with salt instead of heat! Polyelectrolyte complexes were thought to be unprocessable for decades. But we recently found that when you add enough salt you break the ion pairs between polyelectrolytes and you can process them.
Learn MorePolyelectrolyte Multilayers

These are ultrathin films made from water-soluble polymers: negative and positive polyelectrolytes. If you dip a subtrate in these nontoxic polymers alternately, a uniform film builds up.. a few nanometers at a time. The result offers numerous applications.
Learn More