Saloplastics
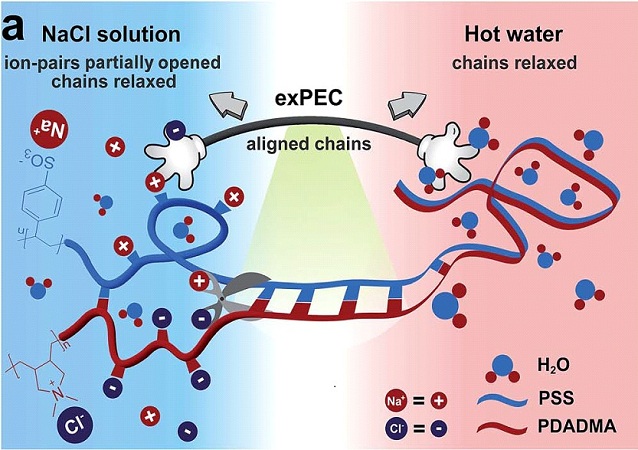
Polyelectrolyte complexes are made by mixing solutions of charged polymers (polyelectrolytes). For example, we use two common polyelectrolytes, poly(diallyldimethyl ammonium) (PDADMA) and poly(styrene sulfonate) (PSS). Because they are brittle and salt-like when dry, polyelectrolyte complexes were thought to be unprocessible for decades. But we recently found that if enough salt is added to break the ion pairs between polyelectrolytes, they can be processed with a regular extruder. The first two photos on the left show salt-plasticized complexes extruded into tapes, rods and tubes. Note that the tube has the dimensions of a blood vessel.
These extruded polyelectrolyte complexes offer a wide range of applications. Adding other materials to the bulk complex before extruding it results in (nano)composites held together by a polymer matrix. We recently mixed iron oxide nanoparticles with PDADMA/PSS and extruded tough, dense fibers which can be heated remotedly by applying radiofrequency fields. (Check out the video of breaking composites with RF)
One of our main interests is the effect of salt on these polyelectrolyte complexes. Observing how they go from being glassy to rubbery to a liquid solution with increasing salt concentrations gives a deeper understanding of the conditions under which these materials are molded and offers new ways of manipulating their properties. The benefits of this area of research are countless as we explore complexes in their solid, liquid and coacervate states.
Selected publications on saloplastics
- J. Fu, Q. Wang, J.B. Schlenoff*; ACS Applied Materials and Interfaces, 7, 895-901 (2015), Extruded Superparamagnetioc Saloplastic Polyelectrolyte Nanocomposites. DOI: 10.1021/am507469
- Q. Wang, J.B. Schlenoff*; RSC Advances, 4, 46675-46679 (2014), Tough Strained Fibers of Polyelectrolyte Complex: Pretensioned Polymers. DOI: 10.1039/c4ra08733j
- R.F. Shamoun, A. Reisch, J.B. Schlenoff*; Advanced Functional Materials, 22, 1923-1931 (2012), Extruded Saloplastic Polyelectrolyte Complexes. DOI: 10.1002/adfm.201102787