Introduction/Background
Elements in the periodic table are generally sorted into two categories: Metals and Non-Metals. Metals are found in the middle and on the left hand side of the periodic table (Groups 1A and 2A and all the B groups). Non-metals are found on the right hand side of the periodic table (Groups 3A-8A).
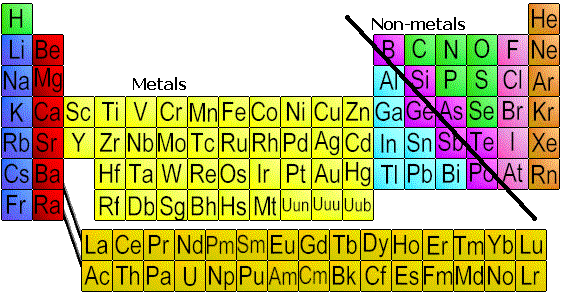
This separation is very important because metals and non-metals not only react differently but they also bond differently when they form compounds. If you know whether the elements in a compound are metals or non-metals, you can then define the compound as covalent or ionic in nature. When metals and non-metals form compounds they form ionic compounds. When non-metals and non-metals form compounds they form covalent compounds. Metals that are combined with other metals are called alloys. Metals are normally a shiny grayish - silver color. There are some exceptions to this such as gold. Can you think of another common exception? Click to reveal
Metals are also harder than non-metals and are good conductors of heat and electricity. Metals have a lot of value in industry because they can be hammered into a sheet because they are malleable and can be pulled into a wire because they are ductile. These physical properties of metals are all due to the arrangement of their electrons in rows that allows the metal atoms to “slide” past each other when necessary.