Hundreds of years ago, Charles I of England hosted a banquet for many of his friends and family. The King's French chef had concocted a new dish. It was cold and resembled fresh-fallen snow but was much creamier and sweeter than any other after-dinner dessert. The guests were delighted, as was Charles, who summoned the cook and asked him not to give out the recipe for this frozen cream. The King wanted the delicacy to be served only at the Royal table and offered the cook 500 pounds a year to keep it that way. Many years later, however, Charles was beheaded in 1649. But by that time, the secret of the frozen cream remained a secret no more. The cook, named DeMirco, had not kept his promise.
In 1774, a caterer named Phillip Lenzi revealed in a New York newspaper that he had just arrived from London and would be offering ice cream for sale. Dolly Madison, wife of U.S. President James Madison, served ice cream at her husband's Inaugural Ball in 1813. Commercial production was begun in North America in Baltimore, Maryland, 1851, by Mr. Jacob Fussell, who is now known as the father of the American ice cream industry.
Key Concepts
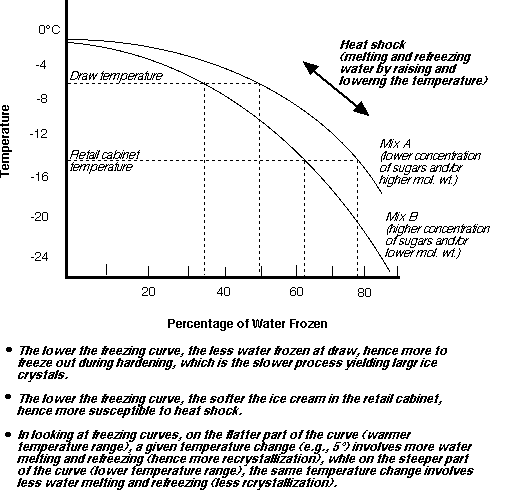
Adding a solute to a solvent lowers the freezing point of that solvent. This change in freezing point is referred to as a colligative property. Freezing point depression of a solution is associated with the number of dissolved molecules. The lower the molecular weight, the greater the ability of a certain mass molecule to decrease the freezing point. When a substance freezes, the particles arrange themselves into an orderly pattern. This arrangement is called a crystal. When sodium chloride is added to the water, a brine or saline solution is formed. The forming of the solution interferes with the orderly arranging of the particles in the crystal. Therefore, more kinetic energy (represented as heat) must be removed from the solvent (water) for freezing to occur. This results in a lower freezing point. Also, the more particles of solute (in our case, the salt) added, the more kinetic energy must be removed. The greater the concentration of solute, the lower the freezing point of the solvent.
During the freezing of the aqueous solution, water freezes out of solution to form pure ice crystals. This causes the freezing temperature of the remaining solution to drop. At temperatures well below the initial freezing point, some liquid water remains. Also, a large increase in the viscosity of the unfrozen phase occurs, thus decreasing the diffusion properties of the system and prohibiting crystallization. It is more difficult to give a freezing time to this process, but it is usually taken as the time to reach some predetermined temperature below the initial freezing point. This freeze-concentration process establishes the freezing curve.
Glossary
Colligative = one of the properties of a solution is a colligative property if it depends only on the ratio of the number of particles of solute and solvent in the solution, not the identity of the solute.
Related Links
What causes an ice cream headache? (http://www.howstuffworks.com/question96.htm)
