
The content that follows is the substance of General Chemistry Lecture 26. In this lecture we discuss the formation of ionic and covalent bonding.
When we discussed bonding before we defined ionic compounds as the compounds that form between metals and non-metals. This is true but now we can look closer at how these bonds actually form and understand more thoroughly why ions form in the first place.
The reason ionic compounds form is that the difference in electronegativity between metals and non-metals is so great that the non-metals simply take the electrons from the metals forming ions of opposite charge. A difference in electronegativity of about 2 will indicate the formation of an ionic rather than covalent bond. This number is a rough guideline not a hard value. Generally our original definition of metals and non-metals making ionic compounds should be maintained.

A couple of notes on the formation of the ions. The ions form such that the electron configuration is made more energetically stable. We said earlier that all ions form to be like the Noble Gases. You can better understand now why this is true. Notice that with the loss of an electron Sodium's electron configuration becomes the same as Neon and with the addition of one electron Chloride's electron configuration becomes the same as Argon's.
Ionic bonds form once the electrons have transferred.

The formation of the bond produces energy called the Lattice Energy. This is the amount of energy released when one mole of ionic compound is formed from gaseous ions.
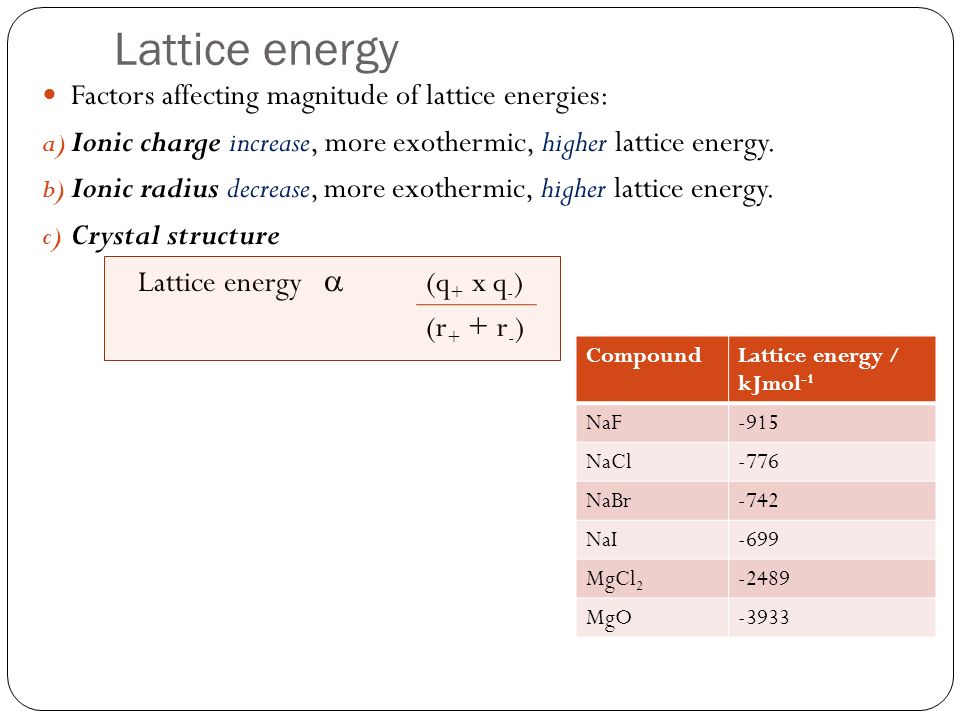
Lattice energies increase with larger charge and smaller radii. The equation for calculating the lattice energy is shown above. q is the charge on the ions and r is the radius of the ion.
Because of this large lattice energy, ionic bonds are very strong. Ionic compounds are solids for this reason as well.
Here is an interactive practice for Ionic Bonds:
Unlike ionic bonds, covalent bonds form from the sharing of electrons. We defined covalent molecules as being made of non-metals. This definition also holds true but again we can now see by use of the electron configurations why these bonds form the way they do.
Valence Electrons
Bonds in Covalent molecules are made using the Valence Electrons. The valence electrons are those found in the outermost shell of the electron configuration. We only discuss valence electrons with respect to the main (A) group elements.
The Noble Gases all have a s2p6 configuration. Because the electronegativities of the non-metals is not sufficient to create ions, the only way for these elements to become like the noble gases is to share electrons to complete their p orbitals.

By sharing the single electron Hydrogen has with another hydrogen or another element it can achieve the 2 electrons that is needed for a full 1s orbital.
For other non-metal elements, the need is to complete the p orbital and achieve a full eight electrons (octet). This need for 8 electrons in the outer shell of an element is called the "Octet Rule".
The number of bonds an element needs to make is based on the amount of shared electrons it needs to complete its octet. For example, Carbon has 4 valence electrons and therefore needs to make 4 bonds. Halogens have 7 valence electrons so they commonly make a single bond to meet the Octet rule.
Here is an interactive tutorial on Covalent Bonding: