Chemical Formulas
This is the 5th lecture for General Chemistry. The topics covered are Empirical and Molecular Formulas, Isomers and the periodic table.
Empirical and Molecular Formulas
When writing the formulas for chemical compounds there are a variety of methods that can be used based on the information you wish to impart:
Molecular Formulas are representations of the molecule that include a listing of both the elements in the molecule as well as the number of those atoms in the formula:
For instance, water has a molecular formula of H2O. This formula reads as two hydrogen atoms and one oxygen atom make up the water molecule. Note that the subscript 2 in the formula indicates the number of atoms for the element it follows.
For example: If the formula was C2H4O2 this would indicate that there were 2 carbons, 4 hydrogens and 2 oxygens in the molecule.
Another way to represent water would be its structural formula: 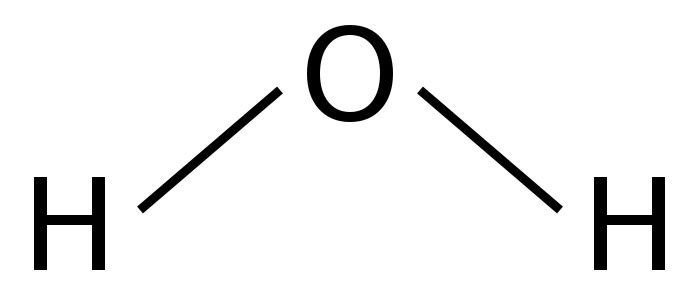
This formula indicates that both of the hydrogens are attached to the oxygen but not each other. Yet another representation of water is called a space-filling model of the structural formula and that would look like this:

And yet another representation of a molecule is what we call an Empirical Formula which simply describes the ratio of atoms in a molecule. This means the representation shows the lowest whole number ratio of the atoms in the molecule:
Later we will use masses and moles to find the empirical and molecular formulas but for now it is just important to know the definitions.
Isomers
Isomer is a fancy word for the different orientations atoms can take in molecules with exactly the same number and ratio of atoms. For instance,
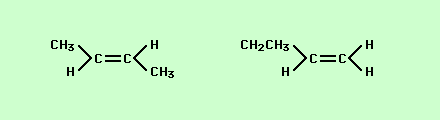
If you look carefully at these two structures you will notice that while the connections between atoms are different the number of atoms and their ratios are exactly the same = C4H8 this makes the two structures isomers. Another type of isomer is one in which the attachments look very similar but vary by how they are attached in space is what is called spatial or geometric isomers,
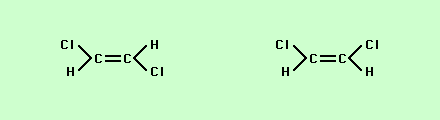
Note that the chloride ions while both being attached to the carbon are either on opposite sides from each other.
The Periodic Table
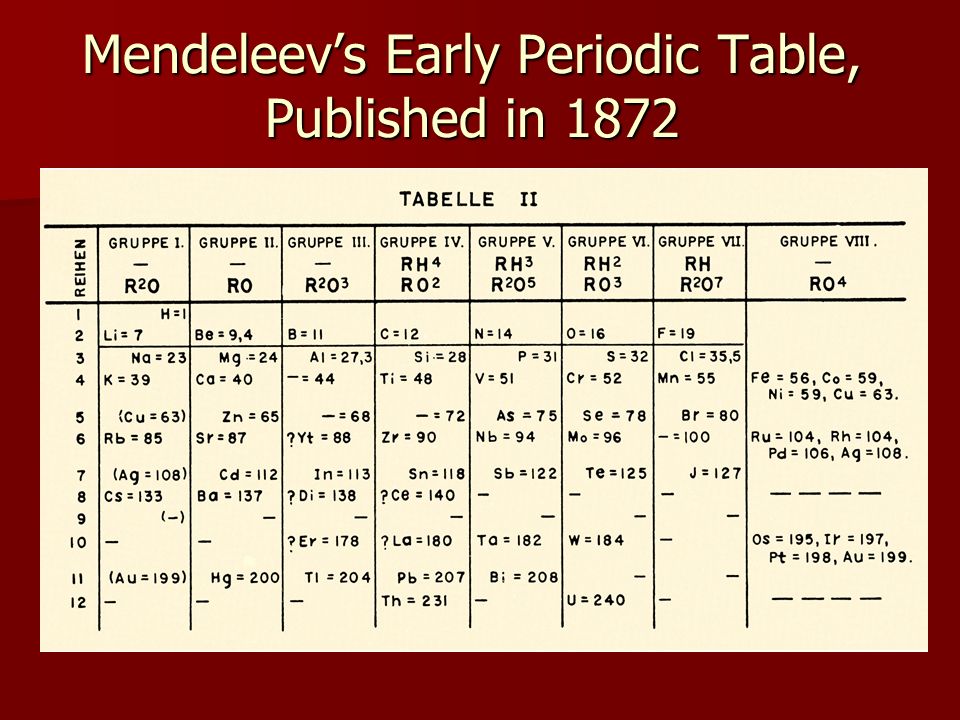
The Periodic Table is a tool that is essential to most chemists. The table lists all of the known elements and even indicates the positions of possible man made elements of the future.
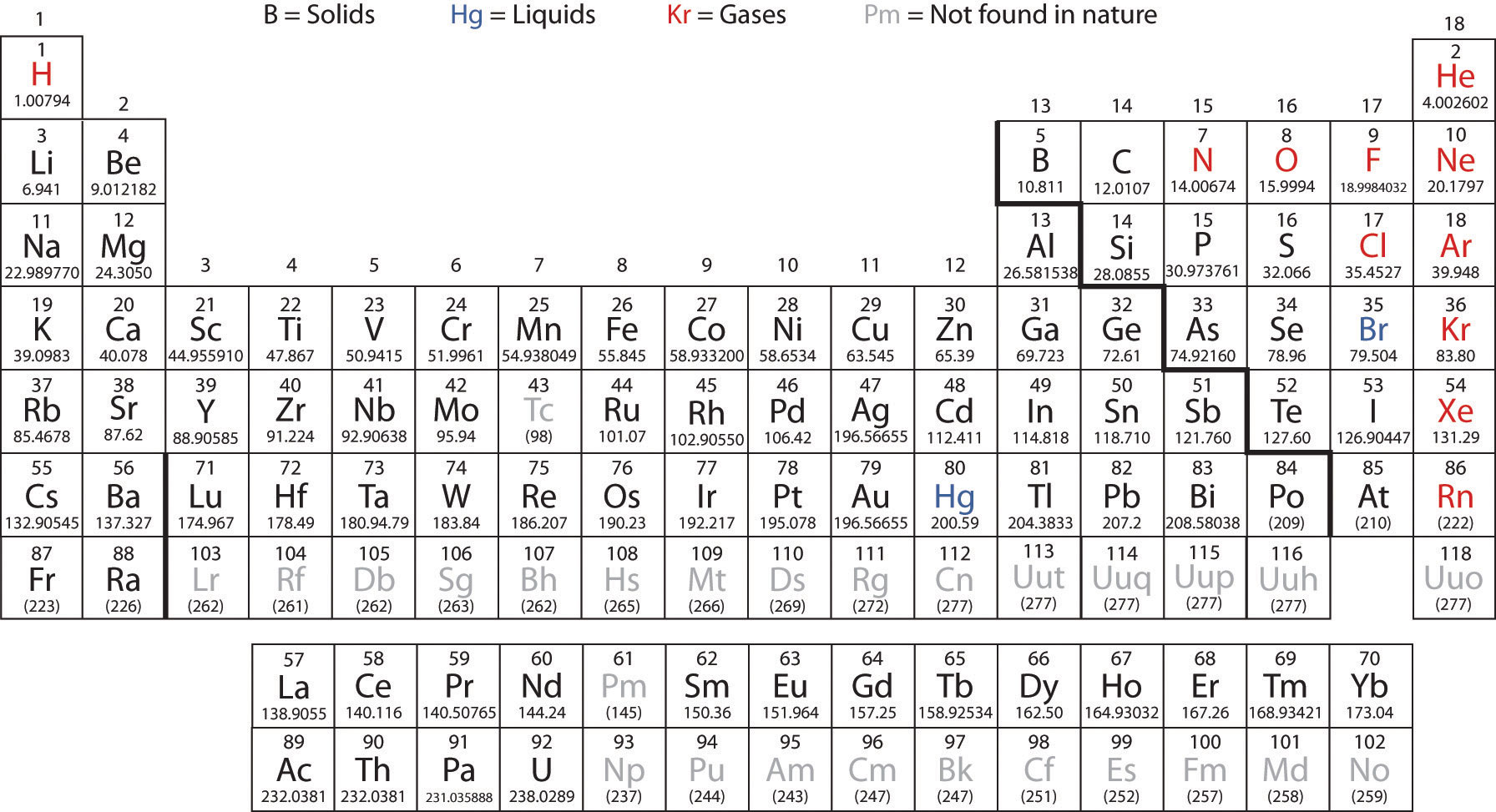
The elements shown in grey are those that are man made. They make these elements by smashing atoms togther inside of nuclear reactors.
For your purposes there are several pieces of information that you can gain from the periodic table that will help you in your calculations as well as determining the type of bonding an element will make.
First let's take notice of the states of most of the elements:
Most are solids. Shown in black. There are only two naturally occuring liquids: Mercury and Bromine. The gases are shown in red and include several first period elements as well as the Noble gases (Group 8).
Rows in the periodic table are called Periods and columns are referred to as Groups. Periods are separated by the way the electrons around an atom fill. We will discuss this in later lectures. For now we will stick to discussing the common features of those elements placed into groups.
Group I is called the Alkali Metals. These metals have similar properties in that they are soft for metals and can be cut with a knife. They are also low density metals and several can float on water.
Group II is called the Alkaline Earth Metals. The reason for this name is their reaction with water:
![]()
The reaction produces a hydroxide base and hydrogen gas. An Alkali is defined as a base that dissolves in water thus these metals are alkaline in their properties. These metals are also softer than most other metals and have low densities.
Groups 3 through 12 are called the Transition Metals. These metals vary in properties somewhat but all are good conductors of heat and electricity. They are not as soft and are more dense than the alkali or alkaline earth metals. They can be hammered or bent into specific shapes and they are less reactive than the alkali or alkaline earth metals. When they do react they often create colored compounds.
Groups 13 and 14 in the periodic table do not have specific titles but rather house the start of the metalloid segment of the periodic table:
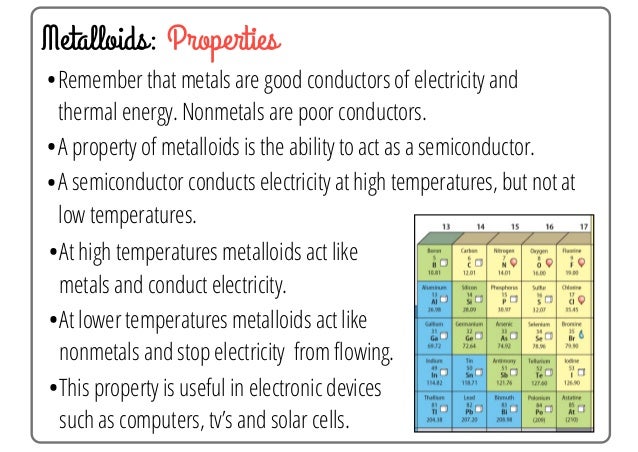
Metalloids (shown in green) are elements that have properties that are both metal and non-metal in appearance. They form the "fence" between metals and non-metals in the Periodic Table and can act similarly to both in reactions.
Group 15 is called the Pnictogen (p is silent) group based on Nitrogen being in the group. The name comes from the Greek word for "choke". This is because pure nitrogen gas has a choking property which is why it is often used for fire suppression. The properties of this group are mixed with some being gases and some being metalloids. The reason they were grouped together was because of their electron configurations (later lectures) and their ability to make -3 ions.
Group 16 is called the Chalcogen Group. The name is also derived from a Greek origin and the elements in the group are called Chalcogens because Oxygen and Sulfur are commonly found in "ores". Chalco means "ore" and gen means "formation". An "Ore" is a naturally occurring solid material from which a metal or valuable mineral can be profitably extracted. Beyond the groups natural affinity to form ores they too were grouped based on their electron configuration as well as the common formation of -2 ions.
Group 17 is called the Halogen Group. The Halogens also have mixed properties with two gases (F2 and Cl2, one liquid (Br2) and the rest solids. They are all diatomic and they have low melting and boiling points but their most important feature is their reactivity with metals forming many of the most common and useful salts (E.g NaCl). They all form -1 ions as well.
Group 18 is called the Noble Gas Group. These gases have also been called the Inert gases since one of their most important properties is that for the most part they don't react with other elements. There are very few compounds that contain a noble gas. They are also all odorless and colorless. They do not form ions.
There are two exerpted rows of the periodic table that if in the table itself would fit in just before the Transition metal block:
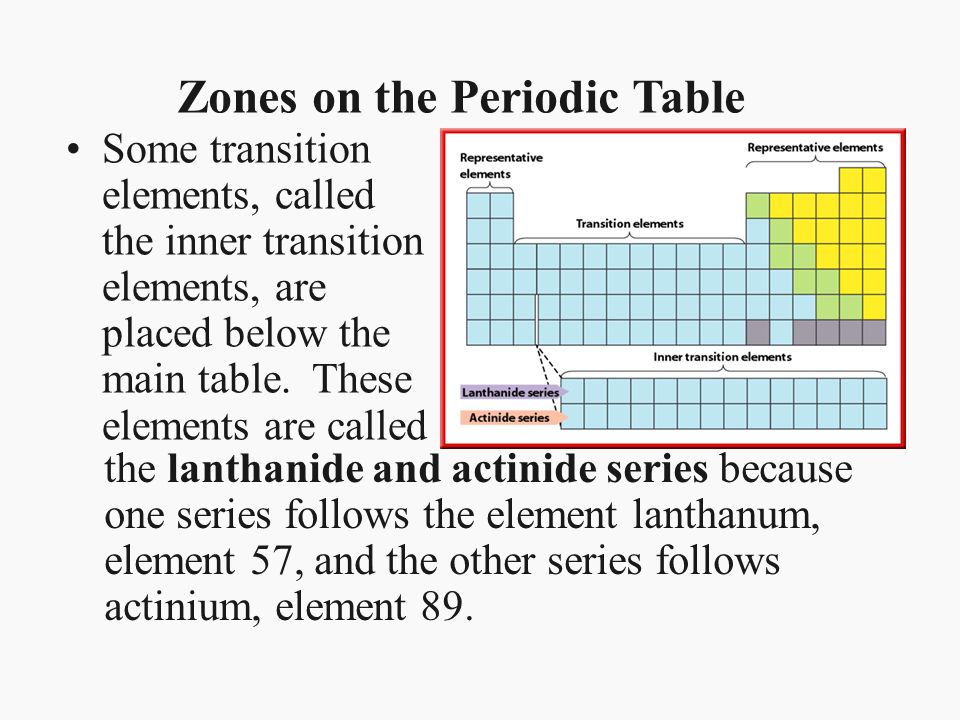
These are the Lanthanides and the Actinides. Together they are referred to as the Rare Earth Metals. They act like most metals in their properties but the actinides have the added property of radioactivity.
You should know the periodic table and most importantly the following segments:

As this knowledge will allow you to determine the type of compound (ionic or covalent) that can be formed.
You should also know the names and symbols for the first 56 elements plus Platinum, Gold, Mercury, lead and Uranium. You can use the flashcards below to help you learn them.
Back to HOME
Back to Segment 1