The content that follows is the substance of General Chemistry Lecture 26. In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements.
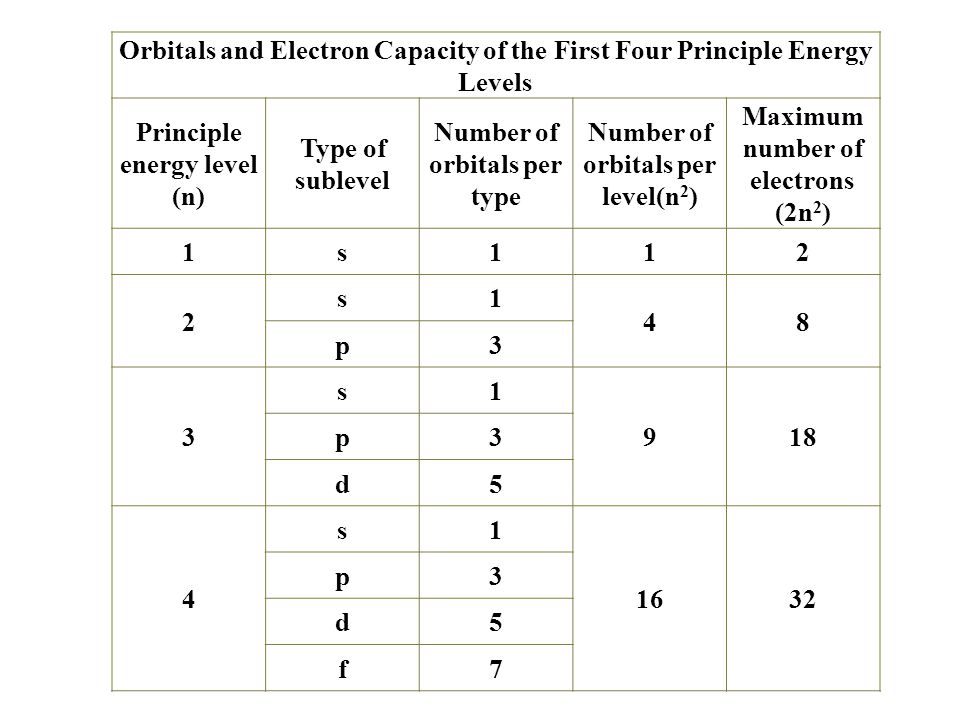
Electron configurations are the summary of where the electrons are around a nucleus. As we learned earlier, each neutral atom has a number of electrons equal to its number of protons. What we will do now is place those electrons into an arrangement around the nucleus that indicates their energy and the shape of the orbital in which they are located. Here is a summary of the types of orbitals and how many electrons each can contain:

So based on what we know about the quantum numbers and using the chart above, you need 2 electrons to fill an s orbital, 6 electrons to fill a p orbital, 10 electrons to fill a d orbital and 14 electrons to fill the f orbital. BUT what we haven't discussed is how these orbitals get filled...the order of fill.
The order in which electrons are placed into the orbitals is based on the order of their energy. This is referred to as the Aufbau principle. The lowest energy orbitals fill first. Just like the quantum numbers themselves this order was determined by calculation and is summarized by the following chart:
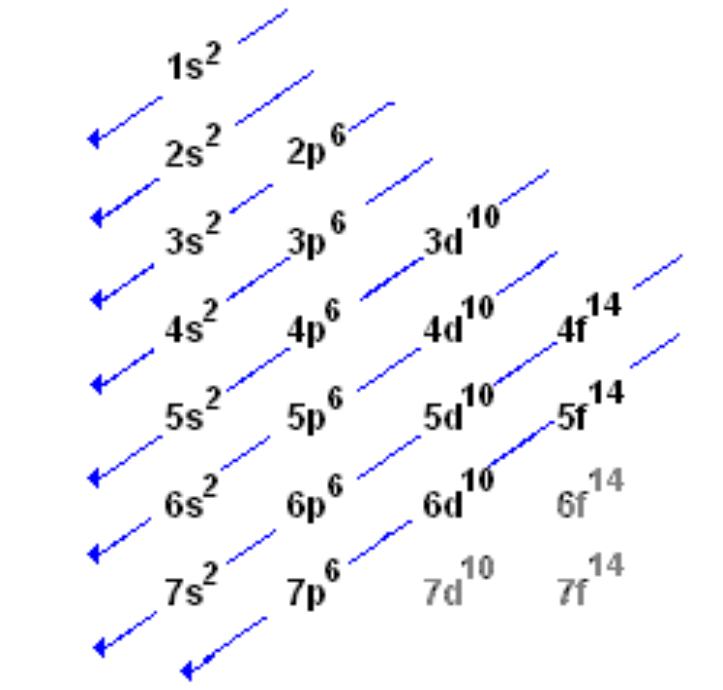
or you can just use the periodic table:
The symbols used for writing the electron configuration start with the shell number (n) followed by the type of orbital and finally the superscript indicates how many electrons are in the orbital.
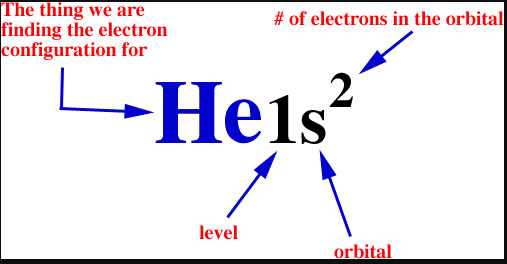
For example:
Looking at the periodic table, you can see that Oxygen has 8 electrons. Based on the order of fill above, these 8 electrons would fill in the following order 1s, 2s and then 2p. So Oxygen's electron configuration would be O 1s22s22p4.
Configurations of ions present a special case of electron configuration and also demonstrate the reason for the formation of those ions in the first place.
If you need to write the full electron configuration for an anion, then you are just adding additional electrons and the configuration is simply continued.
For example, we know that Oxygen always forms 2- ions when it makes an ion. This would add 2 electrons to its normal configuration making the new configuration: O2- 1s22s22p6. With 10 electrons you should note that oxygen's electron configuration is now exactly the same as Neon's. We talked about the fact that ions form because they can become more stable with the gain or loss of electrons to become like the noble gases and now you can actually see how they become the same.
The electron configurations for Cations are also made based on the number of electrons but there is a slight difference in the way they are configured. First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell. Note that this is not always the same way they were added.
Here is an example of what I mean:
Iron has 26 electrons so its normal electron configuration would be: Fe 1s22s22p63s23p64s23d6
When we make a 3+ ion for Iron, we need to take the electrons from the outermost shell first so that would be the 4s shell NOT the 3d shell: Fe3+ 1s22s22p63s23p63d5
One other note on writing electron configurations: A short cut. When writing some of the lower table configurations the total configuration can be fairly long. In these cases, you can use the previous noble gas to abbreviate the configuration as shown below. You just have to finish the configuration from where the noble gas leaves it:

Exceptions
As with every other topic we have covered to date there are exceptions to the order of fill as well. But based on the electron configurations that are generated, these exceptions are easy to understand.
In the d block, specifically the groups containing Chromium and Copper, there is an exception in how they are filled.
Here are the actual configurations:
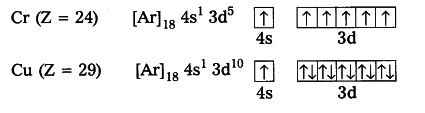
In these columns, the 4s and 3d
There are lots of quizzes on electron configurations you can practice with located here
Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes":

The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but as you can see from above the electrons are placed singly into the boxes before filling them with both electrons. This is called Hund's Rule: "Half fill before you Full fill" and again this rule was established based on energy calculations that indicated that this was the way atoms actually distributed their electrons into the orbitals.
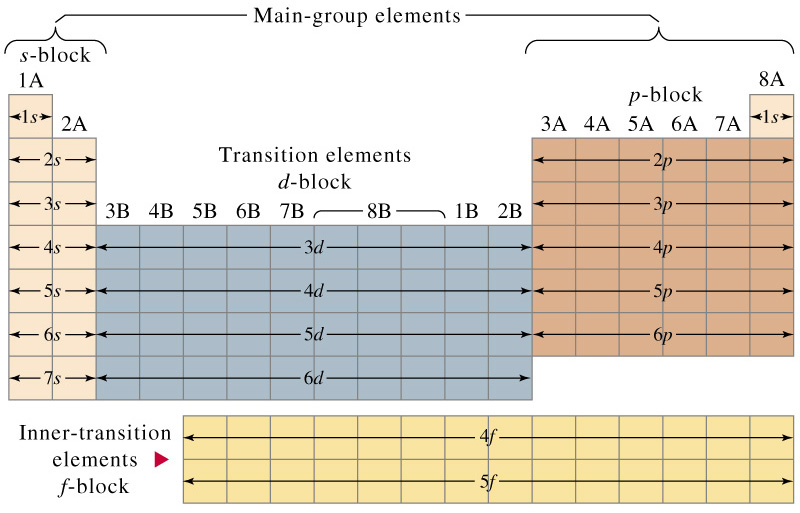
One of the really cool things about electron configurations is their relationship to the periodic table. Basically the periodic table was constructed so that elements with similar electron configurations would be aligned into the same groups (columns).
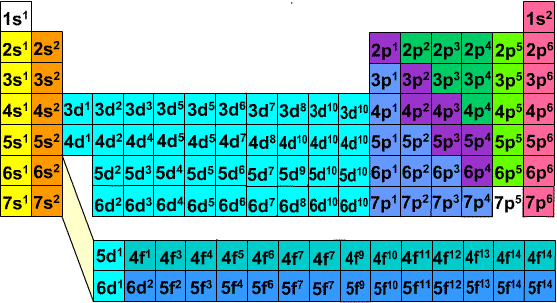
Periodic Table showing last orbital filled for each element
The periodic table shown above demonstrates how the configuration of each element was aligned so that the last orbital filled is the same except for the shell. The reason this was done is that the configuration of an element gives the element its properties and similar configurations yield similar properties.
Let's go through some of the Periodic Properties that are influenced directly by the electron configuration:
The size of atoms increases going down in the periodic table. This should be intuitive since with each row of the table you are adding a shell (n). What is not as intuitive is why the size decreases from left to right. But again the construction of the electron configuration gives us the answer. What are you doing as you go across the periodic table? Answer, adding protons to the nucleus and adding electrons to the valence shell of the element. What is not changing as you cross a period? Answer, the inner shell electrons. So think of it this way, the inner shell electrons are a shield against the pull of the nucleus. As you cross a period and increase the number of protons in the nucleus you increase its pull but since you are only adding electrons to the new shell the shield is not increasing but remains the same all the way across. This means the pull on the electrons being added to the valence shell is increasing steadily all the way across. What happens if you pull harder on the electrons? Well, they come closer to the nucleus and the size of the atom decreases. The effect of the nucleus pulling on the electrons being added across a period is called the effective nuclear charge and is calculated as ZEff = #protons - Core # Electrons. So for example the pull felt by Sulfur would be ZEff = 16 - 10 = +6 |
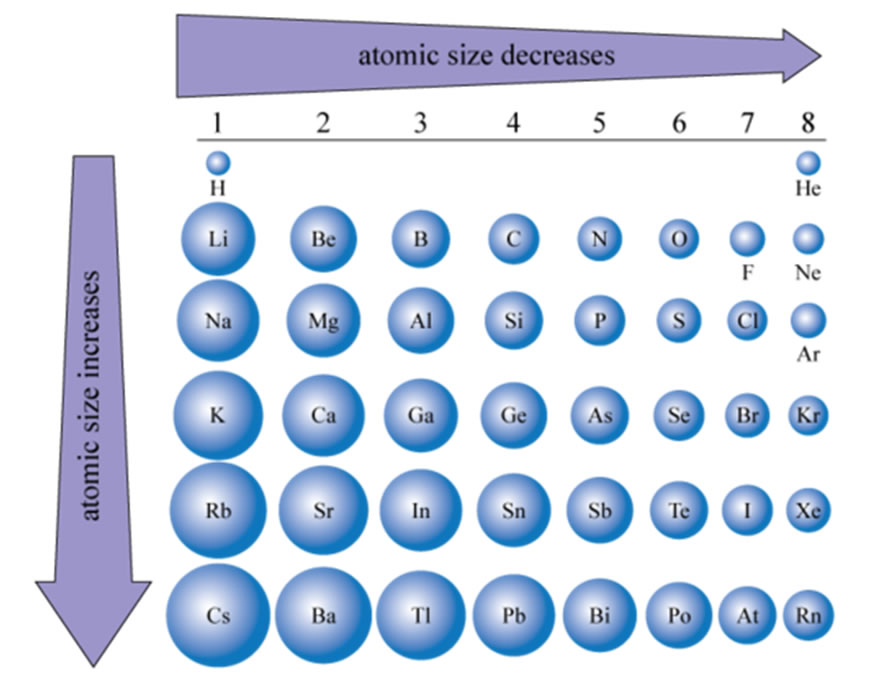 |
Electronegativity may be the most important of the periodic properties you can learn and understand since so many other properties are depend on its value. Electronegativity is an atoms ability to pull electrons towards itself.
Electronegativity is generally expressed by the Pauling Scale and the values were determined experimentally. The table below shows the scale values for the elements.
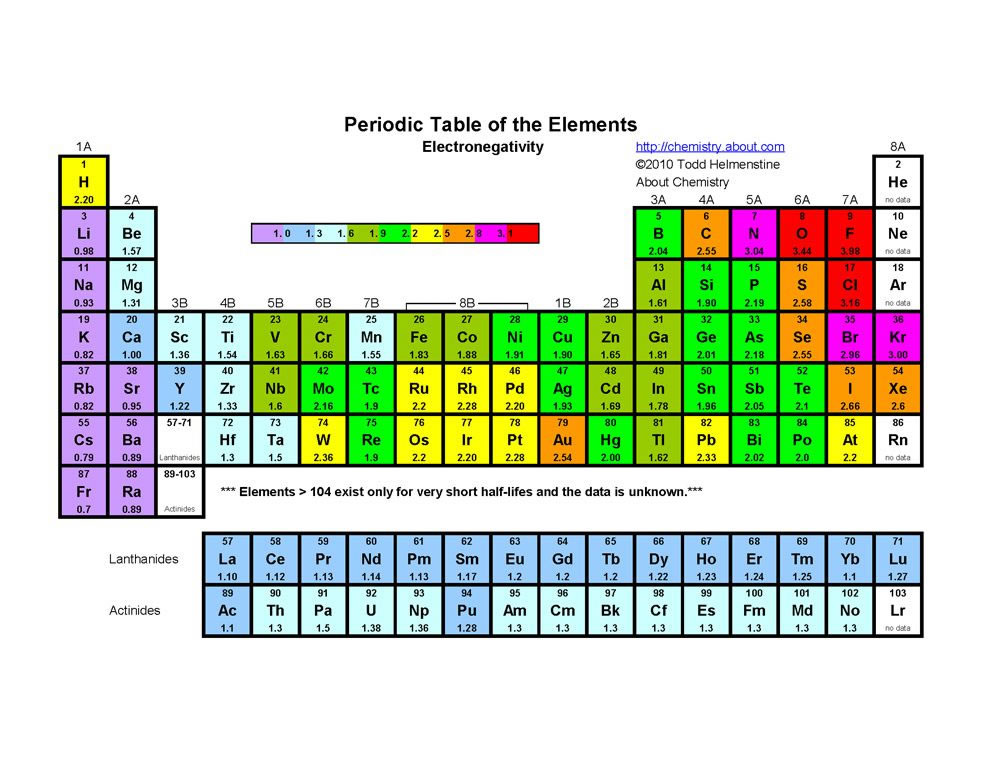
The electronegativity values increase from left to right and bottom to top in the periodic table excluding the Noble gases. The most electronegative element is Fluorine.
From these electronegativity values we can derive the patterns of two other periodic properties: Ionization Energy and Electron Affinity.
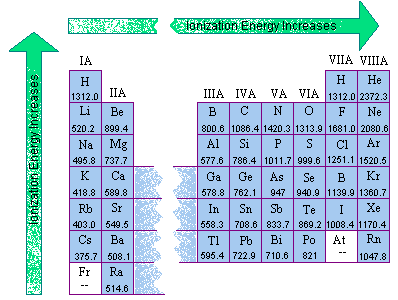 |
Ionization EnergyIonization energy is the amount of energy required to remove an electron from an atom. All ionization energies are positive values because all of these removals (even those for elements that form positive ions) require input of energy. The more electronegative the element, the higher the ionization eneregy. |
Electron AffinityThe Electron Affinity of an element is the amount of energy gained or released with the addition of an electron. The electronegativity and Electron Affinity increases in the same pattern in the periodic table. Left to right and bottom to top. |