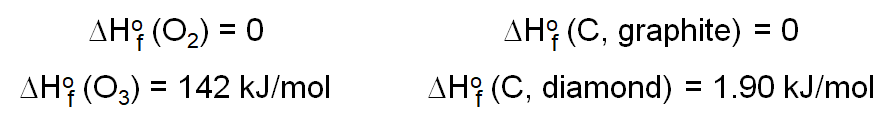
The content that follows is the substance of General Chemistry Lecture 23. In this lecture we further discuss Enthalpy and introduce its calculation using Heats of Formation and Hess's Law.
As we defined it in the previous lecture, Enthalpy is a measure of the heat gained or lost by a system at constant pressure. It is also a state function, meaning its value is only concerned with the current status. The change in Enthalpy is therefore determined by the starting and ending amounts of heat and it does not care how the process was conducted in between these two points. We say that the value is "independent of path"
Further Properties of Enthalpy:
1.Enthalpy is an extensive property. The magnitude of ΔH is dependent upon the amounts of reactants consumed. Doubling the reactants, doubles the amount of enthalpy.
2.Reversing a chemical reaction results in the same magnitude of enthalpy but of the opposite sign.
3.The enthalpy change for a reaction depends upon the state of the reactants and products. The states (i.e. g, l, s or aq) must be specified.
Example Problem using an Enthalpy value:
CH4(g) + 2O2(g) -> CO2(g) + 2H2O(g) ΔH = -802 kJ
Given the above thermochemical equation for the combustion of methane, how much heat energy is released when 4.5 grams of methane is burned (in a constant pressure system)?
First determine the moles of methane: 4.5 g x 1 mole/16 g methane = 0.28125 mol CH4
Then multiply the amount of moles by the known per mole amount of Enthalpy shown: 0.28125 * -802 kJ = -225.56 kJ or -2.3e2 kJ
You may note that the units on the Enthalpy value are only shown as kJ and not kJ/mol in the reaction. This lack of the per mole unit is fairly common but the per mole unit is understood to be there even if it is not written, somewhat like a 1 coefficient in a balanced chemical reaction. Looking at the reaction above you can see that the enthalpy value for the reaction is for 1 mole of Methane or 2 moles of Oxygen or 1 mole of Carbon Dioxide etc.
The Enthalpy values for many substances have already been determined experimentally and are readily available in tables of physical constants. The values are generally taken at what is called "standard state". This is the most stable state of a substance at 1 atm pressure and at a specified temperature, usually 25oC and 1M concentration for all substances in solution.
The thermodynamic standard state is defined so that different scientists can compare results
Standard Enthalpy of Reaction - ΔHoRxn - enthalpy change under standard conditions of 1 atm and 298.15 K.
Be careful not to confuse "Standard State" with STP as these are two different conditions.
There are many other types of Enthalpies as well:
Enthalpies of physical change
One Enthalpy of particular use is the Enthalpy of Formation. The Standard enthalpy of formation (ΔHoF) is the heat change that results when one mole of a compound is formed from its elements (in most stable form/natural) at a pressure of 1 atm. The standard enthalpy of formation of any element in its most stable form is zero.
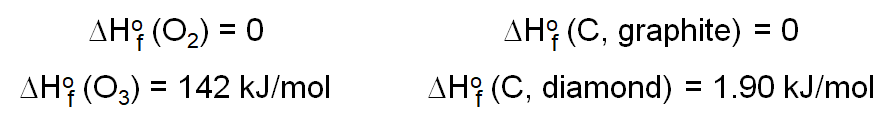
Notice that the elements in their most stable or natural elemental form have a ΔHoF of zero while those forms that are not stable or require a process to form have a ΔHoF value.
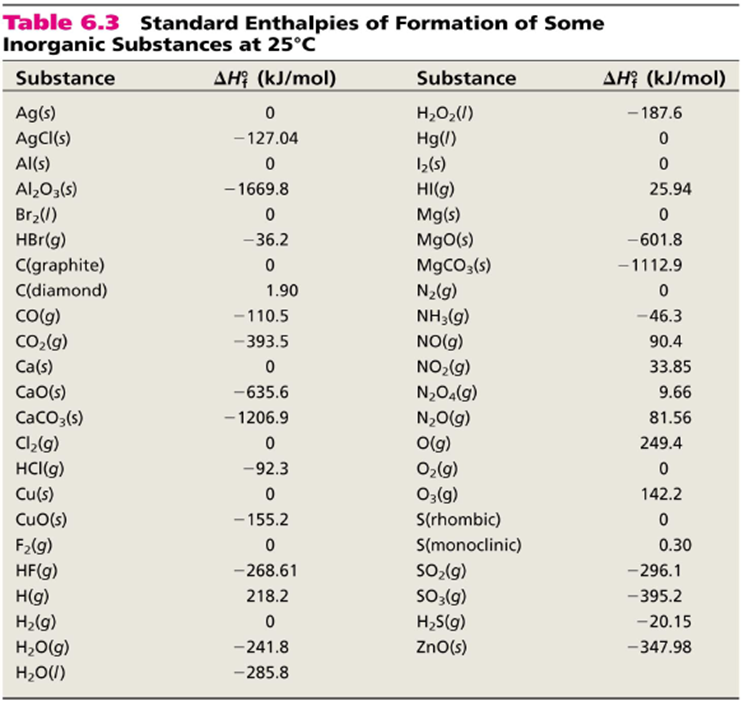
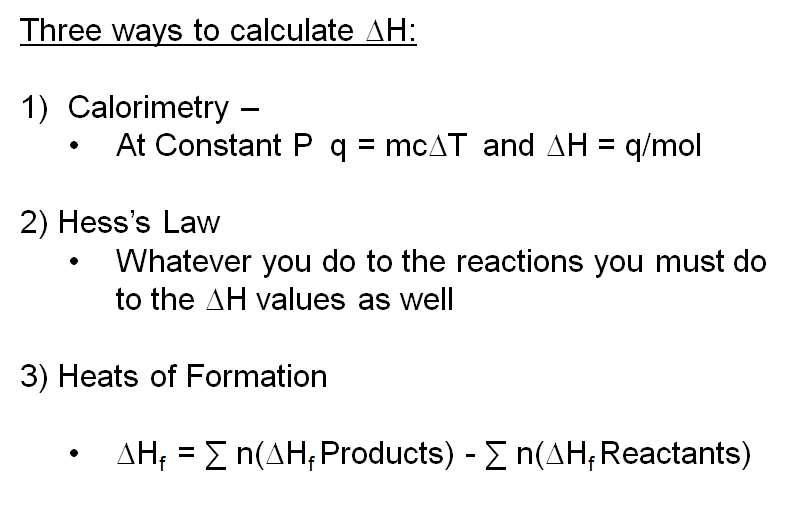
The standard enthalpy of reaction (ΔHoRxn) is the enthalpy of a reaction carried out at 1 atm. We have already learned one process by which we can calculate the Enthalpy of Reaction in Calorimetry. There are two other methods we will learn now:
1) Heat of Reaction from Standard Heats of Formation
2) Heat of Reaction from Hess' Law Calculation
Calculation of the Heat of Reaction from Standard Heats of Formation is based on the following equations:
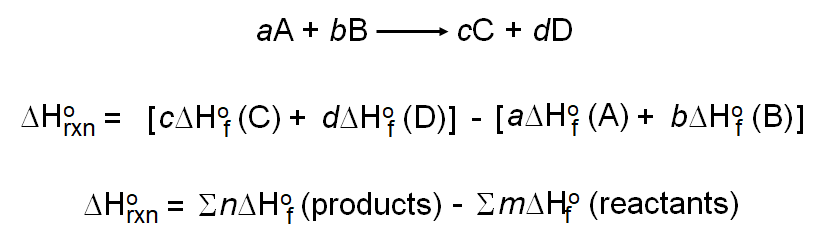
The Heat of Reaction can be calculated from the Heats of Formation of each molecule in the reaction. The equation shown above shows that the ΔHoRxn value is calculated as the sum of the moles of the products times their ΔHoF values minus the sum of the the moles of the reactants times their ΔHoF values.
Hess’s Law: When reactants are converted to products, the change in enthalpy is the same whether the reaction takes place in one step or in a series of steps. Remember that Enthalpy is a state function. It doesn’t matter how you get there, only where you start and end.
Here is an example of how to complete a calculation of this type:
The third process by which you can calculate the Heat of a Reaction is by using a process called Hess' Law. Another way to state Hess' Law is The enthalpy change of an overall process is the sum of the enthalpy changes of its individual steps.
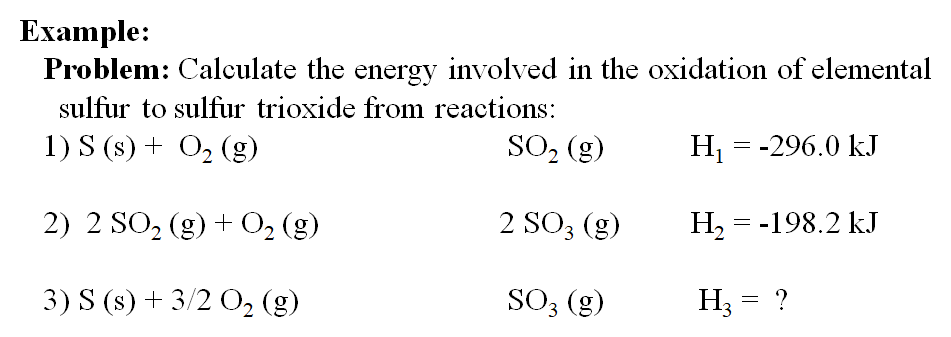
The process needed to answer the question above is based on the principle that if you add two or more equations to get a new equation, you must add the ΔH’s to get the ΔH for the new equation.
A couple of rules before we start:
1) If you multiply an equation by a value to get the number of moles to match the reaction needed, you have to multiply the ΔH by the same value.
2) If you turn a reaction around to get a molecule on the correct side to match the reaction needed, you change the sign of the ΔH value for that reaction.
The "target" reaction is S (s) + 3/2 O2 (g) → SO3 (g) ΔH = ?
In the two reactions we are given, the Sulfur is located in Equation 1 and is in the right form so we just copy that equation in as is. The second equation contains the Sulfur Trioxide we need but in the wrong amount. Since there are two in the equation we will have to divide the entire equation by two to get it into the correct form remembering to cut the enthalpy value in half as well:
S (s) + O2 (g) → SO2 (g) H1 = -296.0 kJ
1/2(2 SO2 (g) + O2 (g) → 2SO3 (g) H2 = -198.2 kJ)
After division the two equations are ready to be added together. We can cancel anything that appears on both sides of the equation in equal amounts:
S (s) + O2 (g) → SO2 (g) H1 = -296.0 kJ
SO2 (g) +1/2 O2 (g) → SO3 (g) H2 = -99.1 kJ
S (s) + 3/2 O2 (g) → SO3 (g) ΔH = -395.1 kJ
Here is a website with lots of practice quizzes on all three types of enthalpy calculations: Here
In Conclusion, at this point we have learned 3 ways to calculate the Heat of a Reaction:
In about a month, once we have learned how to draw Lewis structures you will learn a 4th method using bond energy values.