

Dry Ice
The content that follows is the substance of lecture 17. In this lecture we cover the properties of gases, the units of pressure and the use of a manometer to measure pressure.
We discussed gases as a phase of matter earlier in the course and here we will review those properties in more detail.
A gas has 3 main characteristic properties that separate it from other forms of matter:
1) It is compressible.
2) It expands to fill the container in which it is placed.
3) Its molecules move faster than those of either liquids or solids - meaning it is higher energy.
Let's look at each property separately and make sure we understand its meaning.
1) Compressibility
 |
The ability of a gas to be compressed means we can take a large amount and reduce it into a smaller space to make it easier to transport and use. Propane is a gas we use for both heating and cooking. It is compressed into tanks to make its distribution easier. |
| Thecompressibility ofa gas comes from the large amount of space between the molecules of a gas in its normal state. The compression does not force the molecules to be in contact with each other necessarily, just brings them closer together until they are in a more liquid state than gaseous. If the compression were to continue, the gas could be forced into a solid state. This is how CO2 is formed into dry ice. |
Dry Ice |
2) It Expands to Fill the Container in which it is Placed:
 |
The expansion of gases is easy to understand since the molecules are moving rapidly through space and only turn when they collide with another molecule or object. This means that they will spread until contained. You can smell gases from processes like wood/paper treatment plants or barbeque restuarants from long distances because of this expansion. What this means for a chemist is that the volume of a gas can be assumed to be the same as the volume of the container it is placed in. This makes calculations much easier. |
3) Gases are Higher Energy than Solids or Liquids
Because of the energy of a gas, we often define gases by different physical properties than we use for solids and liquids. Specifically, we define gases by the use of the Pressure they exert on the walls of the container they are placed in, as well as their volume, amount of moles and the temperature of the system. Most of these properties are unnecessary for solids and liquids since their volumes are defined and while they do exert some pressure based on their weight due to gravity it is not changing unlike that of a gas.
There are four properties used to define the state of a gas:
1) Pressure (P)
2) Volume (V)
3) Temperature (T)
4) Amount in moles (n)
Pressure is defined as the force of an object over the area of impact.

The best way to understand this relationship is by thinking about the situation shown below. If you strike both nails shown below with the same force you will get two very different outcomes based on the area of the nail shown. In the case of the nail with the small area (point) faced down, the nail will be driven into the wood because the pressure is greater. In the case of the nail with the larger area the nail would not be driven into the wood but would most likely bounce and hit you (so don't try this at home).
 |
 |
Gases cause pressure as the fast moving molecules continuously strike the walls of the container they are in. The pressure is measured as the average force with which they hit the walls divided by the area over which the force they exert is measured.
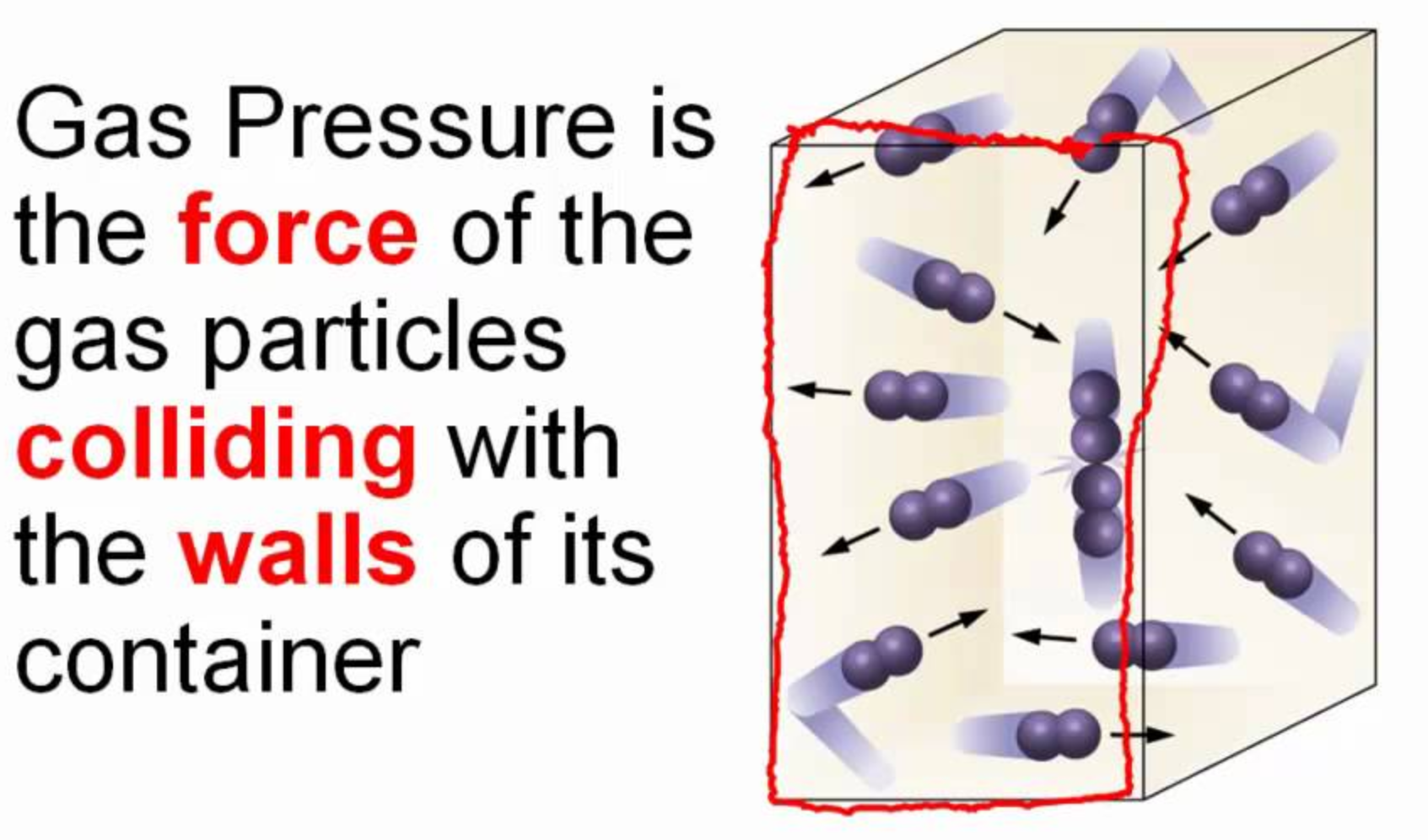
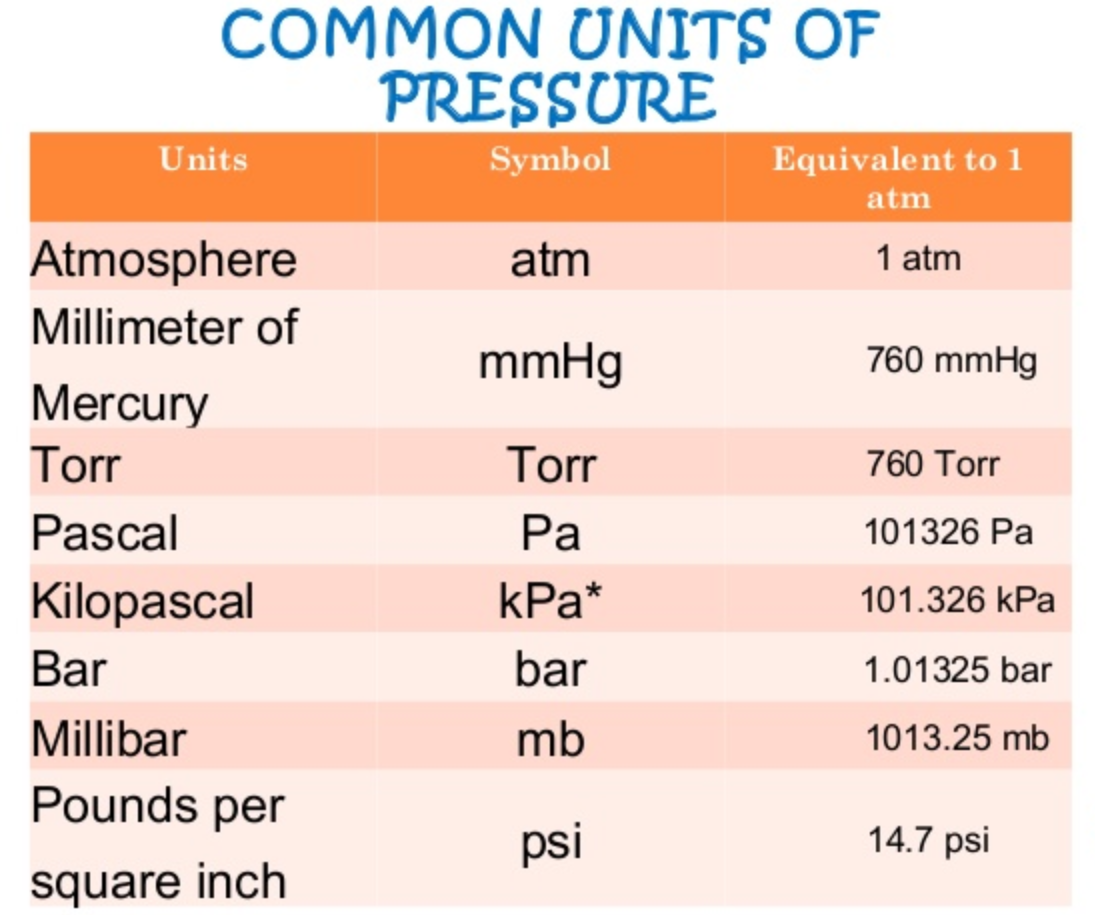
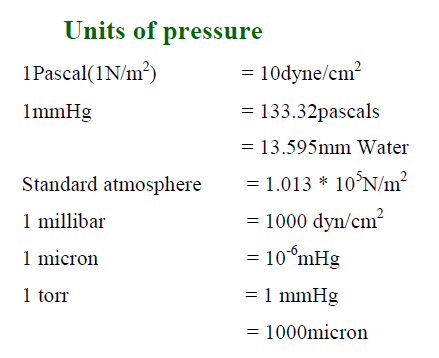
Units of pressure therefore are always in units of force per area. The most commonly used, Atmospheres, is actually equal to 1.013 x 105 N/m2. Below are some of the most commonly used units of pressure. The table on the right shows some of the force/area units they are derived from.
 |
 |
The pressure of a gas is measured experimentally by a device called a manometer. The video below does a good job of explaining what a manometer is and how it is used:
A typical problem you might be asked with respect to the use of a manometer would be the following:
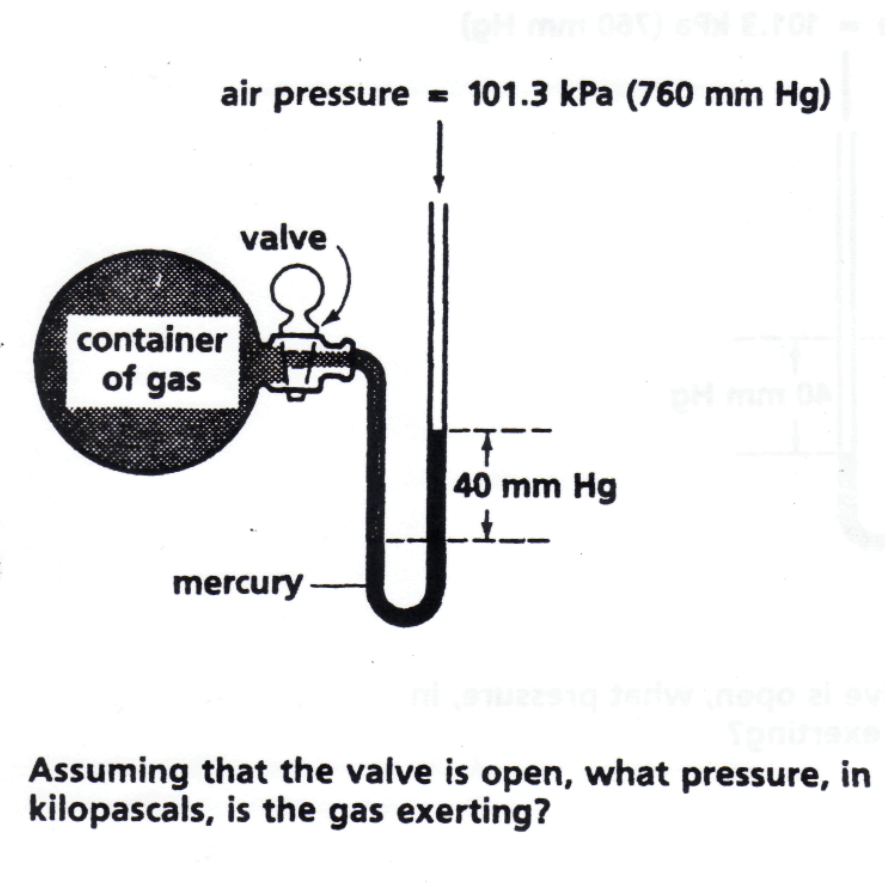
To answer the question, You need to notice the placement of the mercury line. If the gas pressure is greater than the atmospheric pressure you add : 760 mm Hg+ 40mmHg=800mmHg to convert to kPa you just multiply 800 mmHg x 101.3 kPa/760mmHg = 106 Kpa
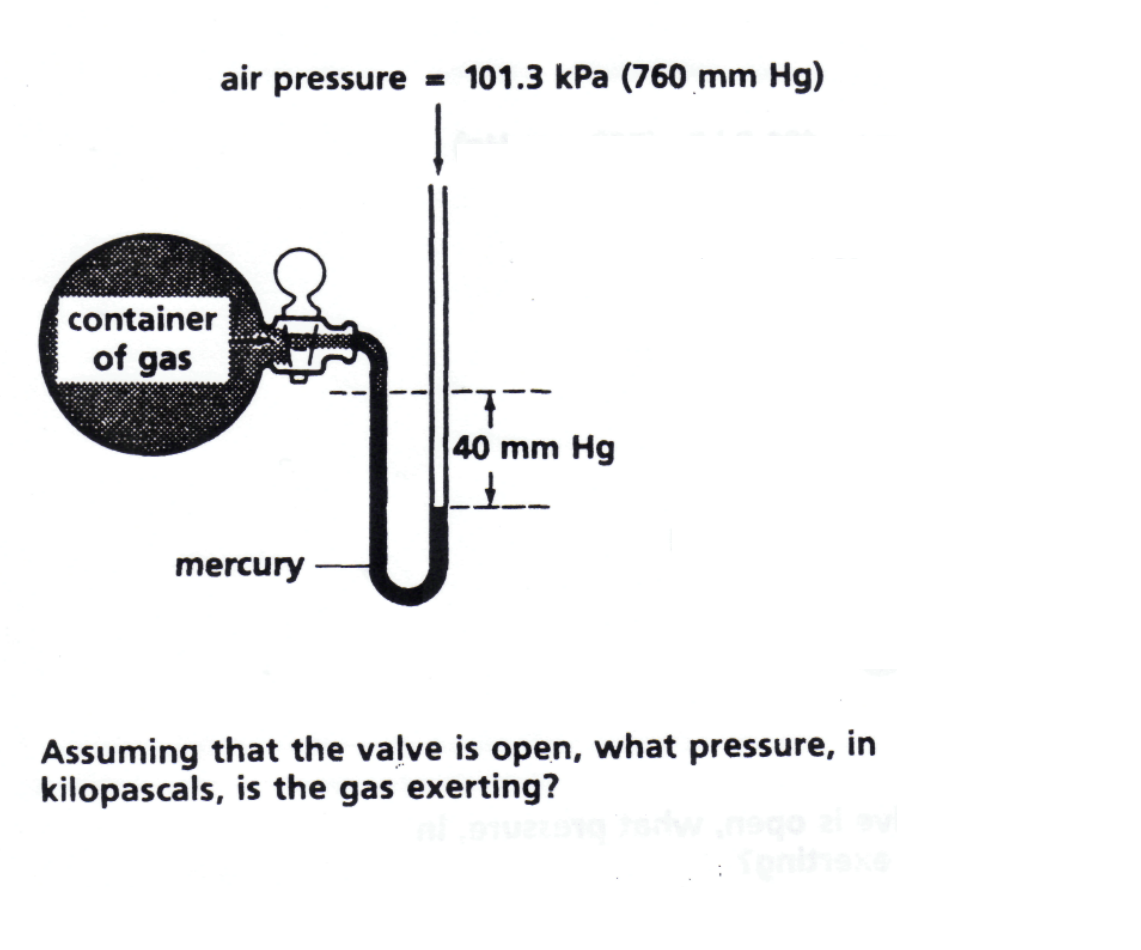
In this case, based on the line of the mercury, you can see that the atmospheric pressure is greater than the gas pressure so you will subtract the difference instead of add: 760mmHg- 40mmHg = 720mm Hg then convert as before: 720 mmHg x 101.3 Kpa/760 mmHg= 95.96 Kpa.
Here are a bunch of questions with the answers for you to try: Manometer Practice
The volume of a gas is really the volume of its container and it will generally be expressed in units of liters(L) or milliliters(mL).
The temperature at which a gas is held has a lot of influence on its other properties. As with increased temperature the gas has increased energy which increases the force with which it strikes the sides of the container which therefore increases its pressure. If pressure is maintained as a constant, then the volume of the container must be increased as temperature increases. Think of the blowing up of a hot air balloon.
The unit for temperature for gases is ALWAYS KELVIN (K) for reasons that will be explained in the next lecture.
The amount of a gas is expressed in moles as represented by the letter "n".
The only other problems associated with this material will be the conversion of pressure units. The need to convert the units will come from two sources, uniformity or matching the units of something called the Ideal Gas Constant which we will present in the next lecture. In either case, you convert the units of pressure the same way you would convert any other units. To practice, just select a value and a unit and then convert to all the rest shown in the tables above. Below is a pressure unit converter you can use to check your answers: