Multiple Bonds
The content that follows is the substance of General Chemistry Lecture 36. In this lecture we continue the discussion of hybrid orbitals and apply this theory to structures with multiple bonds.
Orbital Hybridization
We learned in the previous lecture that Hybridization is the rearrangement of bonding and non-bonding electrons around an atom that occurs during the formation of covalent bonds.
In sp3 hybridization, 1 s orbital electron and 3 p orbital electrons hybridize to form 4 " hybrid sp3 orbital electrons. This allows an atom to make 4 identical length and strength bonds with other atoms. See below:
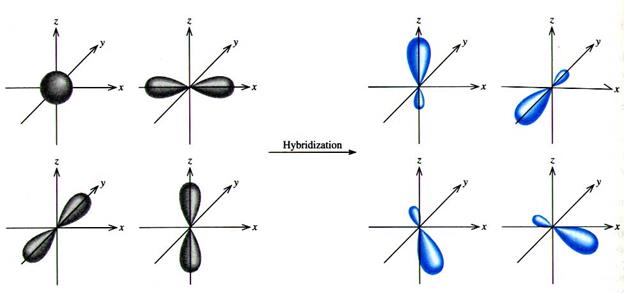
This explains the structure of methane. All of the Carbon-Hydrogen bonds are equivalent:
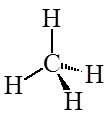
But how does Valence Bond Theory explain multiple bonds? What is and is not hybridized?
The Double Bond
In much the same way we explained the hybridization of carbon making 4 single bonds we can use the same carbon model to show how a double bond will be formed.
We start with the same electron orbital structure that we did for the single bonds: 2s electrons and 2 p electrons. And again we promote one of the s electrons to the p level, BUT when the hybridization takes place only three hybrid orbitals form leaving one electron in an unhybridized p orbital. The three hybrid orbitals are designated as sp2.
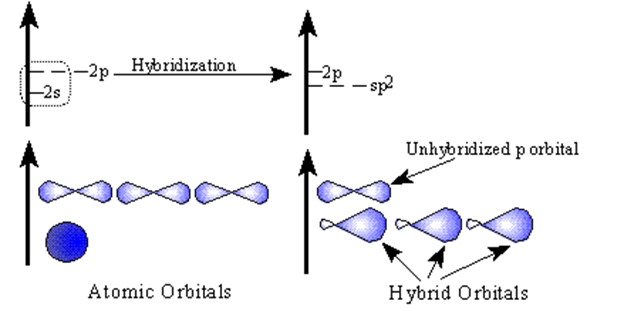
| Sp2 hybrid orbitals are formed from one s and two p orbitals. Therefore, there are three large equal shaped/equal energy lobes. Each lobe points toward the vertex of a trigonal planar structure. The angle between the lobes is 120.0o. |
 |
To show how the bonds are then formed with these hybrid orbitals, let's look at the structure of C2H4:
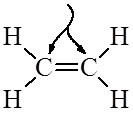
The 4 Hydrogen atoms only have 1 electron each in an s orbital. They form sigma bonds with two of each Carbon's three sp2 hybrid electrons. The third sp2 hybrid electron in each carbon is used to make another sigma between them. Then there is only the unhybridized p orbital electron left over for each carbon. These p orbital electrons form a pi bond between the two carbons completing the double bond.
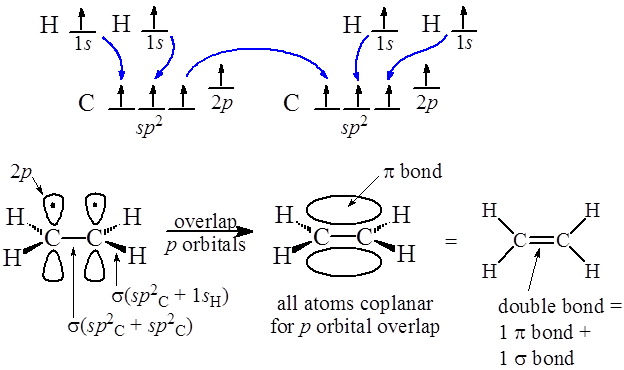
So in summary, a double bond is made up of one sigma and one pi bond as shown above.
Triple Bonds
As you might guess by now the formation of the triple bond is similar in process to the formation of the double bond. But we are now forming an sp orbital formation using carbon as an example so this means we will promote the s orbital electron as always but only two of the electrons will actually form hybrid orbitals which we will call "sp" hybridized electrons.
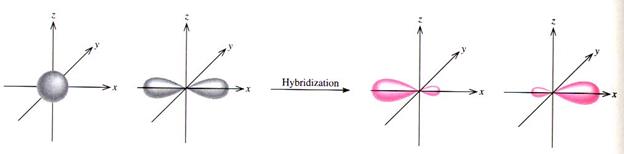
Only two orbitals are hybridized
Sp hybrid orbitals are formed from one s and one p orbital. Therefore, there are two large equal shaped/equal energy lobes. Each lobe points towards each other in a linear structure. |
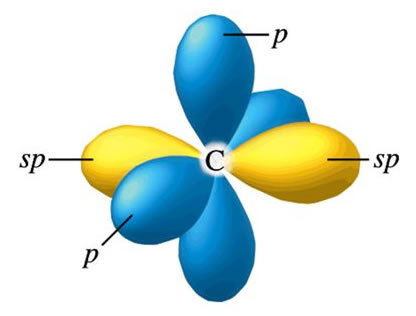 |
This means that there are two unhybridized p orbital electrons with which to make a triple bond. Let's look at Ethyne (C2H2) and follow the bond formation.
![]()
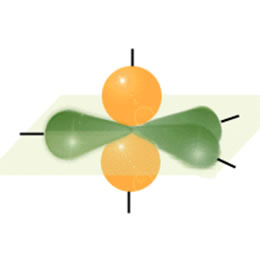
Each Hydrogen will overlap its s orbital electron with one of Carbon's sp hybrid electrons forming single bonds. the carbons then use their remaining sp hybrid electron to overlap with each other forming a sigma bond between them. Finally the two unhybridizes p orbitals will overlap with each other forming two pi bonds as shown below:
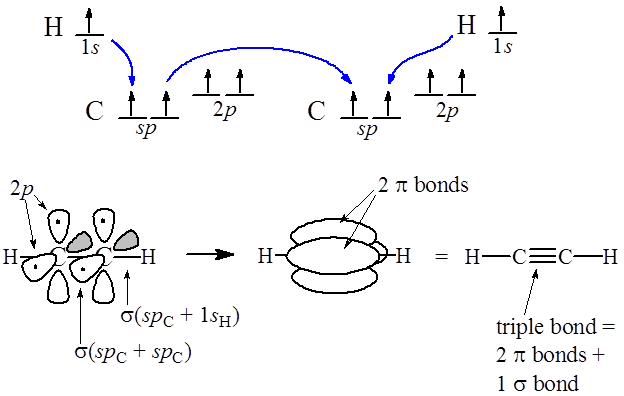
A triple bond consists of a sigma bond (green) (sp overlap) and two pi bonds (red) (p orbital overlap).

Here is a nice summary video that covers all of the material we have discussed in the last two lectures:
Here is a worksheet for you to use for review or practice: Hybridization sample problems
Back to HOME
Back to Final Segment