Types of Reactions
The content that follows is the substance of lecture 12. In this lecture we cover Oxidation Numbers and the Balancing of Oxidation and Reduction reactions by the half reaction method.
Oxidation Numbers
When we talk about why reactions run in the forward direction we generally talk about the production of a product that because it is removed in some way from the solution (gas, solid, covalent system etc.) will not allow the reaction to reverse its direction and therefore the reaction continues to make product.
I often think of this process as a production line with a conveyor belt. As long as the products are steadily being removed from the product side of the belt the production will continue.

But there is another type of reaction that will also push the reactants to products and that is an Oxidation-Reduction (Redox) reaction which is driven by the irreversible transfer of electrons.

I say this reaction is "irreversible" because the spontaneous transfer of electrons only proceeds in one direction. If you wanted to reverse the reaction you would have to input energy.
Another way in which these reactions are different from acid-base or precipitation reactions is that they don't have any obvious indicators of the redox taking place. In other words, there are no redox symbols like there are solid (s) or liquid(l) symbols for the other types of reactions to tell you what they are. The only way to assess if a reaction is a redox reaction is to assign the oxidation numbers to each element and determine if there has been a change.
Assigning Oxidation numbers is done by a set of Hierarchichal rules:
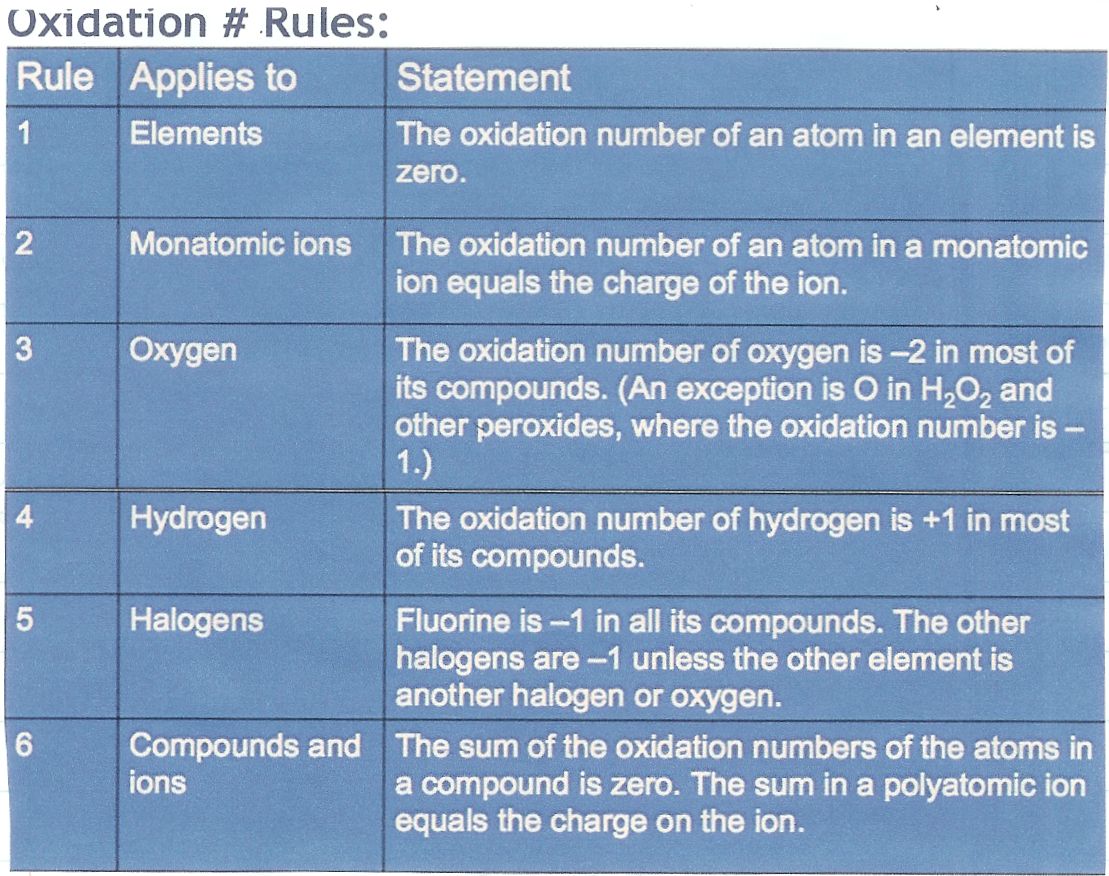
Hierarchichal means that the higher on the list the rules is it supersedes the rules below it.
For the examples below, the oxidation number is written above the element or ion:
 |
Rule 1 applies to both chlorine and hydrogen as they are both in the elemental form. The rule 4 then dictates H is +1. Rule 4 is where you start with C2H6 but then you use rule six to figure out the oxidation number for carbon. Rule 1 again sets oxygen to zero and the rule 3 for oxygen and rule 6 for molecules sets up the determination of the rest of the elements Finally, the last reaction uses Rule 1 for the reactants and then rules 3 and 6 for the product elements. |
When an element loses electrons its oxidation number or oxidation state becomes more positive. This process is called Oxidation. In the reactions shown above, Hydrogen, Carbon and Iron (in Red) respectively are all being oxidized. When an element gains electrons its oxidation number or oxidation state becomes more negative. This process is called Reduction. In the reactions shown above hydrogen, oxygen and oxygen (in Green) respectively are all being reduced.
The simultaneous loss and gain (transfer) of electrons is what defines each of these reactions as a Redox Reaction.
Here is a little more instruction:
AND NOW YOU PRACTICE.....
Balancing Redox Reactions
Just like with the other reactions we have seen to date, a redox reaction must be balanced to be usable. But because we have to consider the balance of electrons being transferred as well as the elements in the reaction there are a different set of steps to create this balance. The process I am going to introduce here is called the half-reaction method and it will be assumed the reaction is being balanced in an acidic aqueous solution.
The last part of the statement above is important because the method we will use assumes a readily available supply of both water molecules and Protons (H+ ions) in the solution.
Here are the steps you will need to use:
i) Assign oxidation numbers to each element.
ii) Divide the reaction into half reactions: Oxidation and Reduction.
iii) Balance the non-oxygen and hydrogen elements
iv) Balance the Oxygen atoms by adding H2O
v) Balance the Hydrogen atoms by adding H+
vi) Balance the charge on both sides by adding e- to the side with the greater positive charge
vii) Multiply the two half reactions by coefficients so that the e-‘s cancel:
viii) Add the two reactions back together and cancel ions etc. that appear in equal amounts on both sides of the reaction:
viiii) Convert the resulting reaction to basic media using H+ + OH- → H2O:
Here is an example of how to complete this process:
Here are some practice problems for you to try:
Practice Problems and Answer Key