Stoichiometry 2
The content that follows is the substance of lecture 15. In this lecture we cover the use of balanced chemical equations to calculate theoretical and percent yields in reactions.
Percent Yield
Whenever you run a reaction, a lot of things have to go right to actually form a product. The reactant molecules not only have to make contact with each other but they also must be aligned properly and also have sufficient energy to make product. Because not all of these things happen every time a reaction is set up, there is very seldom a case where 100% of the reactants become products.
The idea of a 100% yield is called the "theoretical yield" in chemistry because this is the value in mass or moles that could be made if all of the reactants were completely used up. This value is of course limited by the amount of the limiting reactant. So the process we were completing above in calculating the amount of product made by the limiting reagent was in fact the calculation need to determine the "theoretical yield" of a reaction.
The actual yield of a reaction is a measured amount that is taken after a reaction has been completed. In a problem, this value is usually given but may also be calculated so long as you know both the theoretical yield and percent yield of the reaction.
The Percent Yield is defined as
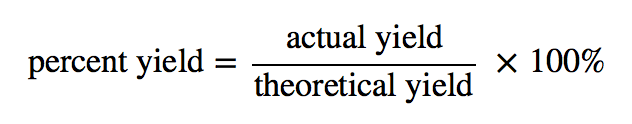
Theoretical Yield Example:
If 4.50 g of HCl are reacted with 15.00 g of CaCO3, according to the following balanced chemical equation, calculate the theoretical yield of CO2. 2HCl + CaCO3 → CaCl2 + H2O + CO2
1. Determine the number of moles of one of the products (CO2 in this example) produced if all of each reactant is used up.
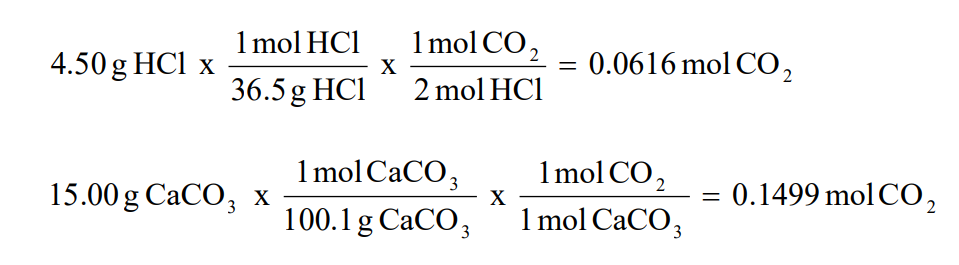
2. Use the smallest number of moles of the product (CO2) from step 1 to calculate the theoretical yield of product (CO2).
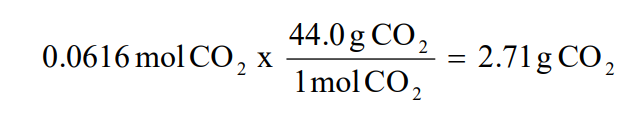
Note: Since the reactant, HCl, produces the least amount of product, it is the limiting reactant and the other reactant, CaCO3, is in excess. Percent Yield Example If 2.50 g of CO2 are isolated, after carrying out the above reaction, calculate the percent yield of CO2.
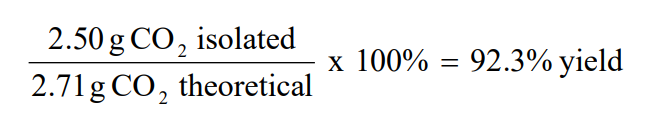
Let's Practice: