VSEPR
The content that follows is the substance of General Chemistry Lecture 33. In this lecture we introduce the shapes and reasoning behind VSEPR.
Valence Shell Electron Pair Repulsion
Valence shell electron pair repulsion (VSEPR) theory is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. The theory centers around the idea that the clouds of electrons that surround nuclei and flow in the bonds that link atoms together repulse each other. Therefore the structures that form from the repulsions will be those that maximize the distances between the atoms and their bonds.
The Theory uses the letter A to represent central atoms and X to represent peripheral atoms and E to represent lone pairs to describe the structure of the molecule.
For Example: The ClO3- Ion has the following structure:
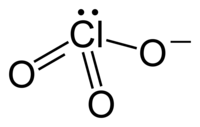
There is one central atom (Cl) and 3 peripheral Oxygen atoms and one lone pair of electrons so the VSEPR code would be AX3E.
Electron Pair Geometry and Molecular Geometry
Electron Pair Geometry (EPG) is the arrangement of the electrons both bonded and lone pairs around a central atom. The Molecular Geometry (MG) is the shape we can "see" of the molecule. This means that the Molecular Geometry only describes the bonds of the molecule and does not describe the lone pair electrons. Electrons do not have sufficient mass for us to be able to see them in the various spectroscopies used to determine molecular structures. We know they are present due to the maintenance of the Electron Pair Geometry but since we can't see them we give a separate name to the structures that have lone pairs present.
Let's go through each of the possible arrangements of electrons around a central atom and discuss both the EPG and MG for each.
AX2
A Central atom with only two other atoms bound to it will form a linear structure in order to maximize the distance between its bonds:
![]()
The angle between the bonds is therefore 180 degrees. If you were to remove one of the atoms forming an AXE type of conformation the structure would remain linear as there is no other structure that would increase the distance between bond and lone pair.
AX3 and AX2E
When there are 3 bonds or lone pairs to the central atom, the EPG and the MG is called Trigonal Planar:
 |
The bond angles that maximize the distance between the atoms is 120o much like a Mercedes Benz symbol.
If we take away an atom and the lone pair of electrons remains, the EPG structure remains trigonal planar but we can no longer see one of the "legs" of the trigonal planar structure and thus we have to rename the MG to reflect the new look:
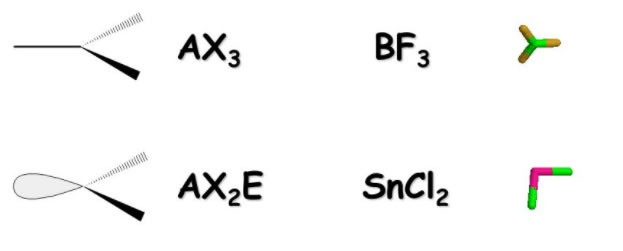
Note that while the structure (MG) that we see does not show the electron pair (Far right above) the shape of the molecule indicates that they are present since if they were not there the stucture with only two bonds would straighten out into a linear form. Because the electrons are present they keep the structure "bent" down as shown and thus this is what the Molecular Geometry of an AX2E structure is called: Bent.
If we were to remove another bond leaving the lone pair there again, the EPG again would remain trigonal planar but all we would see is the single bond and thus the MG would be linear.
AX4, AX3E, and AX2E2
When there are 4 bonds to the central atom, AX4, the EPG and MG are called Tetrahedral:
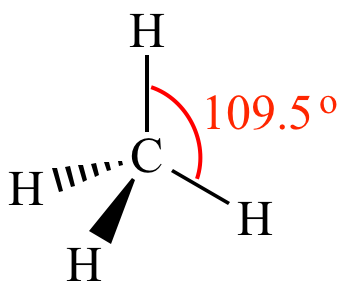
As with the previous structures, as we remove bonds but leave the lone pair electrons in place, the EPG stays the same but the MG changes. For a structure with 3 bonds and 1 lone pair, the EPG is still Tetrahedral but the MG becomes Trigonal Pyramidal (AX3E):
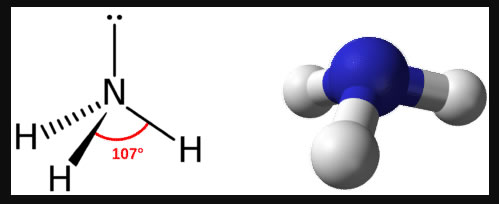
Note that because the electrons in the lone pair are less structured, they influence the structure by repulsing the lower bonds such that the angles are slightly smaller than the normal 109.5 degrees.
If we remove another bond and leave the lone pair of electrons again we find the MG structure is Bent (AX2E2).
Note that again that the presence of lone pairs not in bonds creates repulsion that narrows the bond angle from 109.5 to 104.5.
Any further removal of bonds will leave a linear molecular geometry.
We continue the discussion of VSEPR in the next lecture for those atoms that can exceed the octet rule.
So in summary:
And for practice:
