Review for Exam II
What follows is a step through review of concepts and problems for Chapters 4 and 5. Be sure to TRY the problems on your own before looking at the answers or you are wasting your time.
Concept 1: The balanced chemical reaction
In order to balance reactions, the same number of each element must appear on both sides of the reaction arrow.
Balance the following reactions:
1) ____ N2 + ____ H2 à ____ NH3
2) ____ KClO3 à ____ KCl + ____ O2
3) ____ NaCl + ____ F2 à ____ NaF + ____ Cl2
4) ____ H2 + ____ O2 à ____ H2O
5) ____ Pb(OH)2 + ____ HCl à ____ H2O + ____ PbCl2
6) ____ AlBr3 + ____ K2SO4 à ____ KBr + ____ Al2(SO4)3
7) ____ CH4 + ____ O2 à ____ CO2 + ____ H2O
8) ____ C3H8 + ____ O2 à ____ CO2 + ____ H2O
9) ____ C8H18 + ____ O2 à ____ CO2 + ____ H2O
10) ____ FeCl3 + ____ NaOH à ____ Fe(OH)3 + ____NaCl
11) ____ P + ____O2 à ____P2O5
12) ____ Na + ____ H2O à ____ NaOH + ____H2
13) ____ Ag2O à ____ Ag + ____O2
14) ____ S8 + ____O2 à ____ SO3
15) ____ CO2 + ____ H2O à ____ C6H12O6 + ____O2
16) ____ K + ____ MgBr à ____ KBr + ____ Mg
17) ____ HCl + ____ CaCO3 à ____ CaCl2 + ____H2O + ____ CO2
18) ____ HNO3 + ____ NaHCO3 à ____ NaNO3 + ____ H2O + ____ CO2
19) ____ H2O + ____ O2 à ____ H2O2
20) ____ NaBr + ____ CaF2 à ____ NaF + ____ CaBr2
21) ____ H2SO4 + ____ NaNO2 à ____ HNO2 + ____ Na2SO4
Click Here to check your Answers
Concept 2: Stoichiometry
Once you have a handle on balancing equations you need to know how to use them. When you read a reaction, it gives you information about how the reactants and products are related to one another.
For Example: H2SO4(aq) + 2KOH àK2SO4(aq) + 2H2O(l)
You would read this as one mole of sulfuric acid reacts with two moles of potassium hydroxide yielding one mole of potassium sulfate and two moles of water.
The ratios of reactant to reactant and reactant to product are essentially conversion factors. So if you are given moles of product or reactant you can use these ratios to make predictions.
For example: How many moles of potassium hydroxide are needed to react with 12 moles of sulfuric acid?
12 mol H2SO4 x [2 mol KOH/1 mol H2SO4 ]= 24 mol KOH
Or
How many moles of sullfuric acid and potassium hydroxide are needed to make 5 moles of water?
5 mol H2O x [ 2 mol KOH/2 mol H2O] = 5 mol KOH
5 mol H2O x [ 1 mol H2SO4/2 mol H2O] = 2.5 mol H2SO4
Note that all of the relationships use moles. In order to use the "conversion factors" given in a chemical reaction you must place the amounts of product or reactant into moles first.
Here are some practice problems on calculating molecular weights and converting from grams to moles and moles to grams and using these values to make predictions about product and reactant amounts: PRACTICE
Limiting and Excess Reagents are just a continuation of the use of a balanced reaction to make predictions. There are only 5 questions that can be asked in this type of question:
1) Given an amount of reactant how much product can be made. (This amount is often refered to as the "Theoretical Yield") The reactant that makes the least amount of product or lowest theoretical yield is the limiting reactant. The other reactant will be left over when the reaction is complete so it is the excess reagent.
2) Given an amount of reactant how much of another reactant is needed. You can use the amount of limiting reagent to calculate how much of the excess reagent is used up. The difference between what is used of the excess reagent and what you are given is the amount left over.
3) Given an amount of product how much reactant was used or needed to make it.
4) Given an amount of product what is the percent yield. This problem type requires the completion of problem type 1).
5) A neutralization reaction problem. This problem type requires the completion of problem type 2).
Here is a practice tutorial on limiting and excess reagent problems: PRACTICE
More PRACTICE
Molarity
Molarity (M) is the most common unit of concentration for solutions. It is moles of solute per liter of solution. M = mol/L.
A solute is a chemical molecule dissolved in solution. The solvent is the liquid the solute is dissolved in. The solvent is most commonly water. The solution that has water as its solvent is called an aqueous solution.
Here are some practice problems using solutions rather than grams: PRACTICE
Electrolytes
An electrolyte is any substance containing free ions that behaves as an electrically conductive medium. Because they generally consist of ions in solution, electrolytes are also known as ionic solutions, but molten electrolytes and solid electrolytes are also possible.
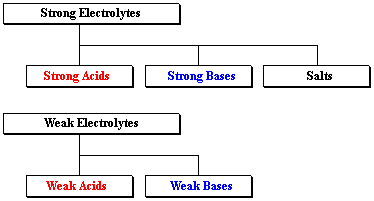
Strong electrolytes fall into three categories: strong acids, strong bases, and salts. (Salts are sometimes also called ionic compounds, but really strong bases are ionic compounds as well.) The weak electrolytes include weak acids and weak bases.

Examples of strong and weak electrolytes are given below:
| Strong Electrolytes | strong acids | HCl, HBr, HI, HNO3, HClO3, HClO4, and H2SO4 |
| strong bases | NaOH, KOH, LiOH, Ba(OH)2, and Ca(OH)2 | |
| salts | NaCl, KBr, MgCl2, and many, many more | |
| Weak Electrolytes | ||
| weak acids | HF, HC2H3O2 (acetic acid), H2CO3 (carbonic acid), H3PO4 (phosphoric acid), and many more | |
| weak bases | NH3 (ammonia), C5H5N (pyridine), and several more, all containing "N" |
Precipitates
A precipitate forms when a reaction between two or more reactants results in the production of an insoluble salt. Precipitates are shown as solids in balanced chemical reactions. In the solution itself they fall to the bottom of the container as the density of the insoluble salt is normally greater than the solvent around it.
The production of a precipitate is one of the reasons a reaction will run in the forward direction (as written) until completion. In order to know if a precipitate will form or not you must know which salts are soluble and which are not.
| Example | Reason | ||
| Na2CO3 | soluble | The sodium cation insures solubility. | Rules #1, 7 |
| CoCO3 | insoluble | The carbonate anion insures nsolubility. | Rule #7 |
| Pb(NO3)2 | soluble | The nitrate anion insures solubility. | Rule #2 |
| K2S | soluble | The potassium cation insures solubility despite the presense of sulfide. | Rules #1, 6 |
| BaSO4 | insoluble | The sulfate anion would insure solubility except that salt has barrium. | Rule #4 |
| PbCl2 | insoluble | Chlorides are soluble, except with lead(II). | Rule #3 |
| (NH4)2S | soluble | Sulfides are insoluble, except with ammonium. | Rules #1, 6 |
| CaCO3 | insoluble | The carbonate anion makes this compound insoluble. | Rule #7 |
| Li2O | soluble | Oxides are insoluble except with group IA cations. | Rules #1, 5 |
| CuSO4 | soluble | Sulfates are soluble. | Rule #4 |
| FeS | insoluble | Sulfides are insoluble. | Rule #6 |
| Pb(C2H3O2)2 | soluble | Acetates are soluble. (Even with lead(II)!) | Rule #2 |
| AgI | insoluble | Iodides are soluble, except with silver(I). | Rule #3 |
| Ni(NO3)2 | soluble | Nitrate insures solubility. | Rule #2 |
| NaI | soluble | Sodium makes this salt soluble, as does iodide. | Rules #1, 3 |
Acids and Bases
Strong acids are acids that are completely or nearly 100% ionized in their solutions. Here are some common strong acids:
Ionization of a strong acid HA can be represented by:
The pH and pOH scale at 298 K |
||
|---|---|---|
| pH | [H+] | pOH |
| 1 | 0.1 | 13 |
| 2 | 0.01 | 12 |
| 3 | 0.001 | 11 |
| 4 | 1e-4 | 10 |
| 5 | 1e-5 | 9 |
| 6 | 1e-6 | 8 |
Solution
The solution of a strong acid is completely ionized. Thus, [H+] = 1.234e-4.
Discussion
What is the pH for a solution containing 1.234 M [HCl]? pH = 0.0913
Solution
The density of such a solution is needed before we can calculate the pH. Since the density is not on the label, we need to find it from the Material Safety Data Sheet, which gives the specific gravity of 1.150. Thus, the amount of acid in 1.0 L is 1150 g.
The amount of HCl = 1000*1.150*0.32
= 368 g (1 mol/36.5 g <- molar mass of HCl)
= 10.08 M
= [H+]
Discussion
Yes, pH have negative values if [H+] > 1.0
| Strong bases | ||
|---|---|---|
| Name | Formula | |
| Sodium hydroxide |
NaOH | |
| Potassium hydroxide |
KOH | |
| Cesium hydroxide |
CsOH | |
| Calcium hydroxide |
Ca(OH)2 | |
Solution
Based on the ionization,
Discussion
The molar solubility of calcium hydroxide is 0.013 M Ca(OH)2. Calculate the pOH. pOH = 1.58
Identifying acids and bases: PRACTICE
Quizzes on Acids and Bases and pH: PRACTICE
Net Ionic Reactions
The presence (or lack) of a driving force for reactions done in water can be evaluated using a net ionic equation. The total and net ionic equations show the real physical state of every component of the reaction. The compound is a strong electrolyte (a soluble ionic compound or a strong acid), then the compound is separated into the appropriate aqueous ions. Otherwise it is left in an undisassociated (ionic compounds) state or un-ionized state (weak acids, bases and water).
Molecular Equation: CaCO3(s) + 2HCl(aq) ® CaCl2(aq) + H2O(l) + CO2(g)
Total Ionic Equation: CaCO3(s) + 2H+(aq) + 2Cl-(aq) ® Ca2+(aq) + 2Cl-(aq) + H2O(l) + CO2(g)
Net Ionic Equation: CaCO3(s) + 2H+(aq) ® Ca2+(aq) + H2O(l) + CO2(g)
The driving force was the formation of water and gas!
Example 2: Fe(s) + AgNO3(aq) ® ?
Molecular Equation: Fe(s) + 2AgNO3(aq) ® 2Ag(s) + Fe(NO3)2(aq)
Total Ionic Equation: Fe(s) + 2Ag+(aq) + 2NO3-(aq) ® 2Ag(s) + Fe2+(aq) + 2NO3-(aq)
The driving force was the transfer of electrons from iron (the reducing agent) to the silver ion (the oxidizing agent). Remember: The reducing agent is the reactant that donates the electrons to the reactant that is reduced. When the reducing agent loses electrons, it is oxidized. The oxidizing agent in the reactant that receives the electrons from the reactant that is oxidized. In doing so, the oxidizer is reduced.
Example 3: K3PO4(aq) + 3HNO3(aq) ® ?
Molecular Equation: K3PO4(aq) + 3HNO3(aq) ® H3PO4(aq) + 3KNO3(aq)
Total Ionic Equation:
3K+(aq) + PO43-(aq) + 3H+(aq) + 3NO3-(aq) ® H3PO4(aq) + 3K+(aq) + 3NO3-(aq)
Net Ionic Equation: PO43-(aq) + 3H+(aq) ® H3PO4(aq)
The driving force was the formation of the weakly ionized acid.
Example 4: KNO3(aq) + HCl(aq) ® ?
Molecular Equation: KNO3(aq) + HCl(aq) ® KCl(aq) + HNO3(aq)
Total Ionic Equation:
K+(aq) + NO3-(aq) + H+(aq)+ Cl-(aq) ® K+(aq) + Cl-(aq) + H+(aq) + NO3-(aq)
Net Ionic Equation: There is no net because all species remained as aqueous ions before and after my hypothesized double replacement reaction. The lack of a driving force leads me t0o conclude that the correct hypothesis is No Reaction.
In summary, there are four reasons a reaction "runs":
1) Creation of a precipitate
2) Creation of a weak electrolyte
3) Creation of a gas
4) change in oxidation of one or more reactants.
Writing net ionic reactions: PRACTICE
Oxidation Numbers
The determination of the oxidation number (or oxidation state) of chemical compounds can be made by following a few simple rules. The rules are presented in order with the previous rule superseding the next.
1. The oxidation number of an atom is zero in a neutral substance that contains atoms of only one element. Thus, the atoms in O2, O3, P4, S8, and aluminum metal all have an oxidation number of 0.
2. The oxidation number of simple ions is equal to the charge on the ion. The oxidation number of sodium in the Na+ ion is +1, for example, and the oxidation number of chlorine in the Cl- ion is -1.
3. The oxidation number of hydrogen is +1 when it is combined with a nonmetal as in CH4, NH3, H2O, and HCl.
4. The oxidation number of hydrogen is -1 when it is combined with a metal as in. LiH, NaH, CaH2, and LiAlH4.
5. The metals in Group IA form compounds (such as Li3N and Na2S) in which the metal atom has an oxidation number of +1.
6. The elements in Group IIA form compounds (such as Mg3N2 and CaCO3) in which the metal atom has a +2 oxidation number.
7. Oxygen usually has an oxidation number of -2. Exceptions include molecules and polyatomic ions that contain O-O bonds, such as O2, O3, H2O2, and the O22- ion.
8. The elements in Group VIIA often form compounds (such as AlF3, HCl, and ZnBr2) in which the nonmetal has a -1 oxidation number.
9. The sum of the oxidation numbers in a neutral compound is zero.
H2O: 2(+1) + (-2) = 0
10. The sum of the oxidation numbers in a polyatomic ion is equal to the charge on the ion. The oxidation number of the sulfur atom in the SO42- ion must be +6, for example, because the sum of the oxidation numbers of the atoms in this ion must equal -2.
SO42-: (+6) + 4(-2) = -2
11. Elements toward the bottom left corner of the periodic table are more likely to have positive oxidation numbers than those toward the upper right corner of the table. Sulfur has a positive oxidation number in SO2, for example, because it is below oxygen in the periodic table.
Definitions: The atom oxidized is the atom whose oxidation number increases. The atom reduced isthe atom whose oxidation number decreases. Half reactions show the gain or loss of electrons by one atom (oxidized or reduced). The oxidizing agent is the reactant containing the atom reduced. The reducing agent is the reactant containing the atom oxidized. Balancing an oxidation-reduction reaction: Find the number of electrons gained and lost by the agents. Select coefficients in front of them so that the numbers of electrons gained and lost are equal.
Assigning Oxidation Numbers: PRACTICE
REDOX
Balancing Oxidation-Reduction Reactions can be done by two methods: 1) Oxidation Number Method or 2) Half-Reaction Method.
1) Balancing a reaction using the Oxidation Number Method:
i) Assign oxidation numbers to each element:
ii) Determine which element is being oxidized and which is being reduced:
iii) Balance the left side of the reaction by multiplying by coefficients:
iv) Balance the rest of the equation:
2) Balancing a reaction by the half-reactions method in both acidic and basic media:
i)Assign oxidation numbers to each element:
ii) Divide the reaction into half reactions: Oxidation and Reduction.
iii) Balance the non-oxygen and hydrogen elements:
iv) Balance the Oxygen atoms by adding H2O:
v) Balance the Hydrogen atoms by adding H+:
vi) Balance the charge on both sides by adding e- to the side with the greater positive charge:
vii) Multiply the two half reactions by coefficients so that the e-‘s cancel:
viii) Add the two reactions back together and cancel ions etc. that appear in equal amounts on both sides of the reaction:
viiii) Convert the resulting reaction to basic media using H+ + OH- = H2O
Balancing Redox Reactions: PRACTICE
Some PRACTICE
More PRACTICE