Lab reports are the synthesis of the work that you performed in the laboratory. An outsider skilled in chemistry should be able to read your report and understand what you did, why you did it and what you discovered. You will find that good written and verbal communications skills are the keys to a successful career. There are many ways to format and present data from laboratory work. The format depends upon the intended audience. A report may be in the form of a publication in a journal or your Ph.D. thesis in a research laboratory. Industrial reports may consist of progress reports, patent applications or analytical results for a client. The emphasis in this laboratory is quantitative chemical analysis. In essence, the instructor (client) needs to know what the results were and with what precision (mean, and confidence interval).
When writing scientific papers you are always continuing the work done by others, building upon the information already provided to you by scientists in both the distant and recent past so it would not be appropriate to write a paper in first or second person (i.e. I did this... or we did this...) since it implies that all of the information is coming from you or you and your partner with no input from outside sources. Therefore when you write a lab report you should always use the 3rd person neutral grammatical person. This means that you do not use any personal pronouns when describing the work or results in the lab and write from an objective point of view.
I. Title Page
Experiment Number and Title
Date Performed__________ Your Name__________
Date Submitted__________ Your Partner's Name__________
Chemistry 1045L-sec#
Lab TA Name
II. Purpose or Abstract
The introduction is often the hardest section to write in a report. A good introduction will explain what the purpose of the experiment was (e.g. measure an unknown value, compare techniques etc…), give a brief background on previous work and offer comments on what is unique or exciting about the work that follows. Think of an introduction like a movie trailer. You are trying to get the reader interested in your work. After all, there are thousands of papers to read, why choose yours? In this class the introduction will be fairly short, normally just a couple of lines will do. Briefly explain the goals of the experiment and the methods to be used. Tell the reader what you are doing, why you are doing it and
Experimental (Procedure)
This section describes the physical work that you performed in the lab. Since the procedures are already written for you it is not necessary to re-write them here. The exception is when you deviate from the published method. Often times seemingly trivial points such as adding B to A instead of adding A to B are critical in explaining anomalous results. You should always include a proper citation of the lab manual in which the procedure or procedures appear.
In MLA style, the components are arranged this way:
Citations for information from the Internet contains as much of the data that you can find to identify the material, as follows in the order given:
You do not need to rewrite the entire procedure. Only describe changes in procedure or specifics like unknown codes that would make your procedure different from what was published.
Data and Results
This section contains, like the title suggests, your results – and that’s it. What it does not contain is an in depth discussion of what the results mean. You should include data tables of raw data where appropriate (see table 1 below) and calculated data and If you pooled data with group members, indicate which data belongs to you and which is from your lab partners. The results section is where you will report the analytical results and confidence interval. If the experiment analyzes an unknown by multiple methods, break the results into subsections according to analyte then method. A sentence such as “The weight percent of KHP in sample # 01-03 was found to be 25.28 ± 0.13 at 95% confidence by titration with standard NaOH.” should be at the end of each subsection. It is also useful to put all of the final results into a table (see table 2 below). This makes it easy for the reader to find the final analytical values and compare methods directly. No calculations, graphs or computations in this section, i.e., only data (numbers and units) as well as the "error" in the result(s).
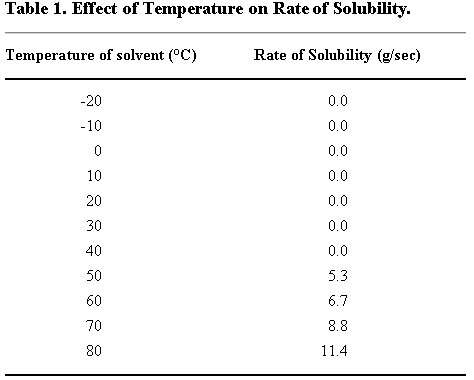
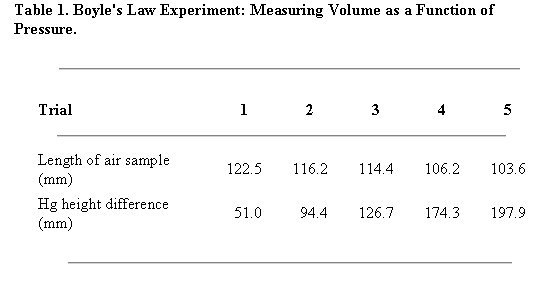
Example Tables 1 & 2:

Calculations
For each different type of result, use illustrations (sample problems) to show how you converted DATA into RESULTS. You are encouraged to use calculators and computers. Computer programs or spreadsheets used in the analysis should appear in this section. Use significant figures in calculations involving measurements. Use units as well as numbers in all calculations. Use dimensional analysis to accomplish this. Write equations of any straight lines from graphs of experimental DATA or RESULTS. In a separate calculation, calculate the % error of each result from the following formula (if required):
% error = (your result - accepted value of result) x 100
accepted value of result
Since graphs are calculated entities those should appear in the calculations section as well.
Conclusion
The conclusion(or discussion) section is a chance to comment on the report as a whole. At this point you may reiterate any key results and discuss their significance. Discuss whether or not the experiment resolved the purpose outlined in the introduction section. Explain any difficulties and how you resolved them during the experiment. There are often questions posed in the lab manual that should be addressed in the conclusion section. Finally, there should be a statement on how "successful" the experiment was in getting the desired result and what possible significant sources of error there might be. You should also discuss possible future experimentation that would overcome any errors or answer any questions left unresolved.