
Background
One of the most important aspects of chemistry is the balanced chemical equation. Recall from the purpose that the law of conservation of mass states that matter can be neither created nor destroyed. Therefore, because matter is composed of atoms that are unchanged in a chemical reaction, the number and types of atoms in a chemical equation must be the same both before and after the reaction.
The Reaction
The experiment you are going to conduct involves the addition of zinc metal (Zn0) to an acidic solution of copper (II) sulfate (CuSO4) which is composed of copper ions (Cu+2) and sulfate ions (SO42-). Thus, the reaction consists of the reactants, species on the left of the arrow: Zn0 (s), Cu2+ (aq), and HCl (aq) in the form of H+(aq) and Cl-(aq), while the products of the reaction, species found to the right of the arrow, are Zn2+ (aq), Cu0 (s), and H2 (g).
You might notice that we don’t mention the sulfate or chloride ions within the reactants or products. This is because in this reaction they are ‘spectator’ ions. They are called spectators because they don’t participate in the reaction we are observing and remain at a constant concentration throughout the reaction. Just as in a math equation, any molecules or ions that remain the same on both sides of the reaction are said to ‘cancel’ each other and can be removed from the net reaction equation. This doesn’t mean those ions are gone, just that we can ignore them since they do not influence the reaction. |
If we know the initial masses of both the solution and the metal pieces before the reaction, we can track how the masses have changed after the reaction, but we should observe that the total mass doesn't change. However, before we can proceed, we need to look at the chemical equations involved so that we can fully understand just what is taking place.
The reaction you will be running can be broken down into the two ‘mini’ net ionic reactions shown below:
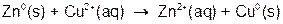
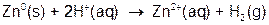
Both of these reactions are referred to as oxidation-reduction reactions. We will return to this subject later, but for now it is simply important to note that the aqueous copper is being converted to solid metal copper. During the reaction you will observe this process by the fading of the blue color characteristic of the Cu2+ ions, and the accumulation of the pink copper metal onto the zinc metal pellets.
Net Reaction
The two ‘mini’ reactions above are then combined, kind of like an addition problem, to form one equation:
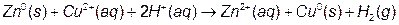
In this equation, you can see that in addition to solid copper (Cu0) being produced and solid zinc (Zn0) being ionized to aqueous Zn2+, hydrogen gas (H2) is released.
Solution Preparation
The first task in this experiment requires you to make 50.0 mL of a 0.50 M solution of CuSO4 in 3.0 M HCl. Because this is one of the first solutions you will be preparing this semester, what will follow is a tutorial-like discussion of the proper method for preparing solutions.
As an example, let’s pretend that we have to make 50.0 mL of a 0.75 M solution of potassium permanganate (KMnO4) in 3.0 M HCl. The first step in this process is to obtain about 50 mL of the 3.0 M HCl in your 100-mL graduated cylinder. With the HCl obtained, we have to calculate how many grams of KMnO4 we will need to make a 0.75 M solution. In order to do this, we first have to find the number of moles in our solution and then multiply by the formula mass of KMnO4.
The formula mass for a compound is calculated by adding together all of the atomic masses found in the formula. For KMnO4 this is the mass of 1 potassium atom + the mass of 1 manganese atom + the mass of 4 oxygen atoms:
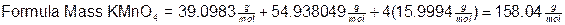
Molarity is defined as the number of moles of solute divided by the liters of solution. Note that we said liters of solution not just liters of solvent. This means that the total solution volume (including solute volume) is in the denominator (M = mol/LSoln).
For our solution, the solute is the potassium permanganate. Since we know the molarity of the solution is supposed to be 0.75 M and the volume is supposed to be 50.0 mL, we can calculate the number of moles of KMnO4 needed:
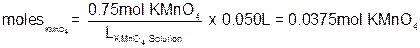
The hardest part is now done! With the number of moles of KMnO4 known, all we have to do is multiply by the molecular weight of potassium permanganate to get the number of grams.
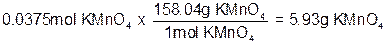
We now need to prepare the flask in which we will make the solution. Solutions are normally made in specially calibrated volumetric flasks. For our solution, we will use a 50.0 mL volumetric flask, filling it about 2/3 full with the 3.0 M HCl we are using as the solvent for our solution. Note that you should never completely fill the flask before adding the solute. This is because addition of the solute will affect the overall volume of the solution and you don’t want to exceed the 50.0 mL total volume. Next, we weigh out the 5.93 grams of KMnO4 using the proper weighing technique, and add this amount to the 3.0 M HCl in the flask. After stirring, we can add as much of the remaining HCl required so that the final total solution volume is 50.0 mL. We will use this same process to produce the solution needed to perform this week's experiment.
Volume of H2 Evolved
As stated above, the reaction we will be observing produces hydrogen gas. In order to determine that the mass of reactants and products has been conserved, we need a method by which we can collect and determine the mass of this hydrogen gas. In order to accomplish this task successfully, you will use a side-armed Erlenmeyer flask:

and a balloon. By sealing the end of the balloon to side-arm, all of the gas released from the reaction will fill up the balloon. We can then determine the volume of the balloon by measuring its radius (r), and height (h) if necessary, and substituting them into one of the following equations:
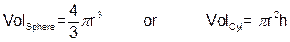
Assuming the temperature of the gas is approximately room temperature, 298 K (or 25 oC) and that the atmospheric pressure is 1.00 atmospheres, you can calculate the mass of hydrogen gas by substituting the volume of hydrogen gas in L into the following equation:
Mass of H2 gas (in g) evolved = 8.244 X 10-2 x (volume in L)
[Reminder: 1 cm3 = 1 mL = 0.001 L]
|