








|

Background
One of the most useful things about studying chemistry is discovering the amount of useful information contained in the periodic table. Many of these periodic properties of the elements are discussed in your textbook.
In today’s experiment, some of the properties of the Group 7A (Group 17) elements, known as halogens, and their compounds will be explored. The solubility properties of the halogens will be used to observe their reactions.
The more electronegative an element is, the more it attracts electrons. Group 17 atoms that have become ions by gaining an extra electron, such as F–, Cl–, Br–, and I–, are called halides. Note that the chlorine atom in NaCl, sodium chloride, is a halide—specifically a chloride. Also note that anions such as halides must always be paired with cations when found in the formula for a binary ionic compound. Group 17 atoms in their natural diatomic state, such as F2, Cl2, Br2, and I2, are called halogens. In this experiment, the relative electronegativities of the halogens will be determined.
If a solution containing halide, X–, is added to a solution of a different halogen, Y2, there are two possibilities. When element Y is more electronegative than element X, Y2 will take the electron from X–, leaving X2 as a halogen. On the other hand, when Y2 is less electronegative than X–, no reaction will take place, and Y2 remains as the halogen. In terms of balanced equations:
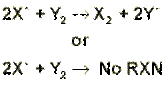
Polar and Non-Polar Solvents
The properties of two solvents, water and hexane, will be useful in sorting out what happens in this type of reaction. Water is a polar solvent and it will solvate polar species whether they are ionic or molecular. This means that a polar molecule (one that has a dipole moment) or an ionic compound may dissolve in water. A diatomic molecule is polar if the two atoms have different electronegativities. Identify which of the halogens and halides in the above equations are ionic and which are non-ionic.
Non-polar solvents solvate non-polar molecules. Hexane is an organic molecule that is non-polar. Since water is polar and hexane is non-polar, the two do not mix. When combined, two distinct, colorless layers are formed with water, the denser liquid, on the bottom.
If colored substances are added to a test tube containing water and hexane, the polarity of the compounds can be determined. If they are non-polar, they will color the hexane layer. If the colored substances are polar, the color is observed in the water layer.
For the first reaction described above, if Y2 is green before any reaction takes place, the hexane layer is green because non-polar compounds will reside in the hexane layer. As the reaction proceeds, the green disappears from the hexane layer because the Y2 molecules are reacting and disappearing. The hexane will then take on the color of X2. In the second case, where X is more electronegative than Y, the more electronegative atom already has the electrons, so no reaction will occur. Since no reaction occurs, the hexane layer will remain green, the color of Y2.
Whenever a color change occurs, this is a clue that a reaction is taking place. Three halogens in aqueous (water) solutions will be available: chlorine, bromine and iodine. Each of these halogens has a distinctly different color in hexane. Therefore, by observing the color of the hexane layer, the halogen present can be determined.
|