








|

Background
Many of the concepts used in this experiment, including limiting and excess reagents, moles, and theoretical yields are covered extensively in your textbook. Since the treatment is extensive, another deep discussion here would be redundant, so we will just hit the highlights.
Limiting and Excess Reagents
The concept of limiting and excess reagents deals with how much product results when two or more reactants are mixed. This concept has very practical applications in the real world. For example, in the business of manufacturing a chemical product of some kind, it is important to do it as efficiently and with as little waste as possible. This is also true in the laboratory, where you want to use only the amounts of reactants necessary to produce the largest amount of product, as anything more would be simply wasted.
Consider a simple reaction where one mole of reactant A reacts with one mole of reactant B:
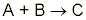
What happens if too much reactant A is added? When all of reactant B is used up, there will be some reactant A left over. In scientific terms, we say that reactant B is the limiting reagent. In other words, no matter how much A is added, no more product is made when B is consumed—reactant B limits how much product is obtained. On the other hand, reactant A is called the excess reagent because there is more than enough of reagent A.
In the reaction above, if we add more of the limiting reagent, reactant B, more of product C is formed. Why, you may ask? Well, reactant B limits how much product is obtained, so when more B is added, the reaction will resume until one of the reactants is used up again. Therefore, the final question is “What is the most efficient mixture of these two reactants?” In fact, the best mixture consists of correctly matched amounts of reagent A and reagent B that would allow for both reactants to be completely used. Note that unless the mole ratios are one to one, “correctly matched” does not mean “evenly matched”.
Finding the Limiting Reagent
For practice, let’s look at the following example and see if we can determine which reagent is limiting and which is in excess. Let's assume we have time-warped to the early 1970s where we all are employed by NASA as rocket scientists. We are working with a fuel mixture composed of dinitrogen tetraoxide (N2O4) and hydrazine (N2H4). These two reagents react to produce nitrogen gas (N2) and water vapor (H2O), as shown below. We have been assigned to figure out which reactant is limiting when 1.40 kg of N2H4 and 2.80 kg of N2O4 are allowed to react.
N2H4(l) + N2O4(l) à N2(g) + H2O(g)
The first step is to balance the equation:
2N2H4(l) + N2O4(l) à 2N2(g) + 4H2O(g)
Next, we have to use stoichiometry to see how many moles of one of the products are produced based on the initial amounts of each reactant. Let’s find the number of moles of H2O.
Based on moles of Hydrazine (N2H4)
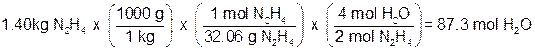
Based on moles of Dinitrogen Tetraoxide (N2O4)
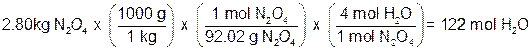
Since hydrazine produces fewer moles of water, it must be the limiting reagent!
As you can now see, it is very important to calculate exactly how much product can be made based on the stoichiometry of each and every reactant. Another point to note is that in any reaction there is always a portion of the product that is lost to human error, incomplete mixing of reagents, etc. Therefore it is important in any chemical preparation to take this loss into account so that enough product is made.
Precipitation
In this experiment, the reaction of the two solutions, copper (II) nitrate, Cu(NO3)2, and potassium iodide, KI, forms a precipitate. A precipitate is a solid formed during a reaction between two aqueous compounds. (This is the same word meteorologists use when talking about falling rain or snow.) In order to better separate the solid and liquid phases, test tubes containing precipitates are placed in a centrifuge. By spinning them around at an extremely high velocity, the centrifuge forces the heavier precipitate to the bottom of the test tube. After centrifugation, a solution that was cloudy has become clear with a solid accumulated at the bottom of the tube
.
The clear liquid found above the precipitate is called the supernatant. In this experiment, we will test the supernatant to see which reactant remains after the reaction is complete. The reactant that is present in the supernatant when the reaction stops is obviously in excess, while the reactant not detected in the supernatant was completely used and is in fact our limiting reagent. This can be verified by adding more of the two solutions we are studying. Using our example earlier, if more solution A is added and additional precipitate forms, then reagent A is the limiting reagent. Similarly, if more solution B is added and additional precipitate forms, then reagent B is the limiting reagent.
The Reaction in the Experiment
Specifically, this experiment involves mixing aqueous solutions of Cu(NO3)2 and KI, which will react as shown:
2Cu(NO3)2(aq) + 4KI(aq) à 2CuI(s)↓ + I2(aq) + 4KNO3(aq)
Since we are dealing with solutions, the concentration is expressed in molarity, a term used in our Conservation of Matter experiment. Recall that molarity (M) is defined as the number of moles of solute in one liter of solution. If we know the volume of solution we can then calculate the number of moles present by multiplying the volume in liters by the molarity of the solution:
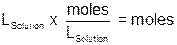
A reaction between 0.50-M Cu(NO3)2 and 0.50-M KI now has a little more meaning to us. In the experiment, each student will be assigned different volumes of reactants to investigate. Based on the volume of reactants assigned, a theoretical amount of product can be calculated. After the solutions react, the precipitate is recovered and its mass determined. The final step in the process is to evaluate the overall efficiency of the reaction by reporting the percent yield of the reaction based on the experimental results. |