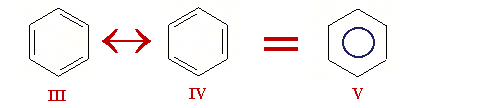
What are aromatics ? Aromatics, so called because of their distinctive perfumed smell, are substances derived from crude oil and, in small quantities, from coal. Aromatics are hydrocarbons, organic compounds that consist exclusively of the elements carbon and hydrogen – without which life would not be possible on Earth. The main aromatics are benzene, toluene and the xylenes; they are used as starting materials for a wide range of consumer products. Many items taken for granted in our everyday lives rely on products made by the aromatics industry, with benefits like durability, safety, comfort and lightweight design. Aromatics are used to make products for areas as diverse as medicine, hygiene, transport, telecommunications, fashion and sports. Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. It can also be considered a manifestation of cyclic delocalization and of resonance.
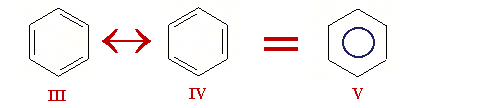
Characteristics of aromatic compounds include:
1) Must be Cyclic
2) Must have (4n + 2) pi Electrons (n = 1,2,3,4,...)
3) Resist Addition but Prefer Substitution
4) Must Possess Resonance Energy
Examples of aromatic compounds:
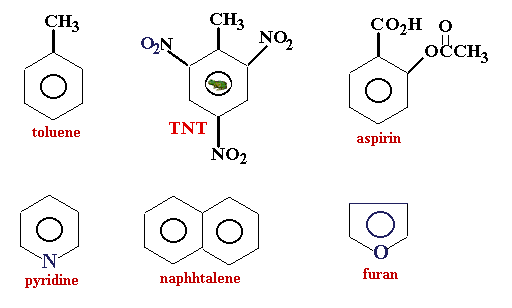
Nomenclature of Aromatic Hydrocarbons
Benzene is the most common aromatic parent structure.
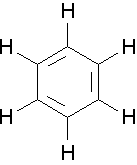
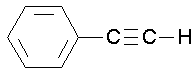
a. The benzene ring is named as a phenyl group when it is a substituent.
phenylethyne
b. Multiple substituents on a benzene ring are numbered to give these substituents the lowest possible numbers. When only two substituents are attached to a benzene ring, they can be named by the common nomenclature using ortho (o-) (1-2 placement), meta (m-) (1-3 placement) or para (p-) (1-4 placement).
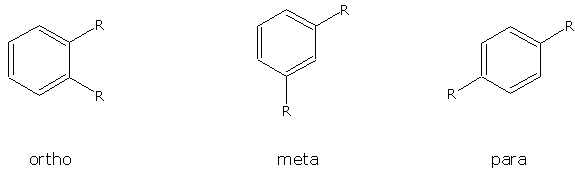
Try to name the following compounds using both conventions...
Try to draw structures for the following compounds...
1,2,4-trimethylbenzene Answer p-dimethylbenzene Answer
c. Some common names involving benzene rings that you should know are...
toluene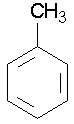 ortho-xylene
ortho-xylene 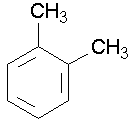
Try to draw structures for the following compounds...
meta-xylene Answer para-xylene Answer