Molecular Orbital Theory is primarily used to explain the bonding in molecules that cannot be explained by Valence Bond Theory. These are molecules that generally involve some form of resonance. Resonance implies that a bond is neither single nor double but some hybrid of the two. Valence bond theory only describes the bonding of single or double or triple bonds. It does not provide an explanation for resonance bonding.
Molecular orbital theory does describe resonance.
The Rules of Molecular Orbital Theory:
First principle: The number of molecular orbitals produced is always equal to the number of atomic orbitals brought by the atoms that have combined.
Second principle: Bonding molecular orbitals are lower in energy that the parent orbitals, and the antibonding orbitals are higher in energy.
Third principle: Electrons of the molecule are assigned to orbitals from lowest to successively higher energy
Fourth principle: Atomic orbitals combine to form molecular orbitals most effectively when the atomic orbitals are of similar energy.
OK. So what do those principles actually mean???
Principle 1: Example - Hydrogen ( H2 ) Each hydrogen atom has a single valence orbital, this being the 1s orbital. Two molecular orbitals
may be formed by the constructive and destructive overlap of these two atomic orbitals. So if you have two 1s atomic orbitals you can only make two molecular orbitals from them. This is the First Principle.
According to MO Theory, the two molecular orbitals that form are called s (sigma = bonding) and s* (sigma star = antibonding). In the case of H2 both of the valence electrons that form the bond between the hydrogens fill the bonding or s orbital.
Principle 2 & 3: This interaction of atomic orbitals, which gives rise to the molecular orbitals, may also be represented in the form of an orbital (electron) energy diagram which shows the relative energies of the orbitals. In the specific case of hydrogen each of the isolated atoms has one electron in its 1s orbital and when the atoms combine to form H2 the two electrons may be accommodated (with opposite spins) in the bonding molecular orbital, as illustrated below. The second principle explains why electrons would want to fill molecular orbitals in the first place. As you should know by now, stability comes from lowering energy needs. Think about it. Don't you feel better when your energy demand is lowered? If not, I would be happy to increase your homework? Anyway...because the bonding molecular orbitals provide a lower energy, more stable state for the electrons, they fill these orbitals first. This also explains the third principle statement as well.
Principle 4: If you note in H2 we combined two 1s orbitals to form a single lower energy s molecular orbital. The fourth principle states that stable molecular orbitals are easiest to form when constructed out of atomic orbitals of similar energies. This means that 1s orbitals should combine with 1s orbitals and 2p orbitals should combine with 2p orbitals etc. to form the most stable molecular orbitals.
Filling up the Molecular Orbitals:
We Start with:

We then form Molecular Orbitals:
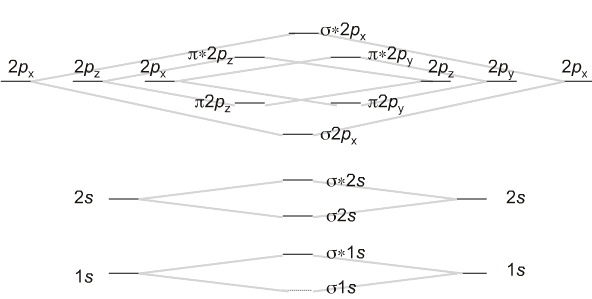
Which fill from lowest to highest energy as follows:
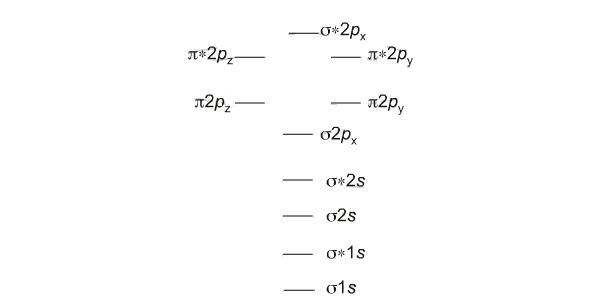
The bond order for a molecule can be determined as follows: bond order = ½ (bonding electrons − antibonding electrons). Therefore, the H2 molecule has a bond order of ½ (2 − 0) = 1. In other words, there is a single bond connecting the two H atoms in the H2 molecule. In the case of He2, on the other hand, the bond order is ½ (2 − 2) = 0. This means that He2 is not a stable molecule.
Let's try an example: N2
Each Nitrogen atom has 7 electrons and an electron configuration of 1s2 2s2 2p3 . This yields a total of 14 electrons to work with. How many molecular orbitals can form? Which molecular orbitals will form? Which orbitals are filled first? What is the bond order for N2?
Something to notice:
1) Since dinitrogen completely fills the s1s and s*1s orbitals they cancel each other out and it becomes more obvious as to why it is the valence electrons that actually control bonding. As you continue from period to period in the periodic table this trend of cancellation amongst the core electrons continues.