Contining on from CHM1045 there are several topics that you must have a firm grasp on in order to be able to understand the concepts being presented in CHM1046.
1) The structure of the atom
2) Valence electrons
3) Lewis Structures
4) Ions and salts
5) Polarity
Let's start with the first of these topics: The structure of the atom.
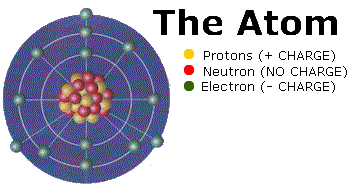
An atom is made up of protons, neutrons and electrons. Protons and Neutrons are located in the nucleus of the atom and electrons are located in shells surrounding the nucleus.

An elements atomic number is equal to the number of protons located in its nucleus. If you change the number of protons, you change the element you are talking about. The atomic mass of an element is equal to the mass of its protons plus its neutrons. From the mass in the periodic table and the atomic number, you should be able to determine the number of neutrons in the atom.
Example:
Oxygen has an atomic number of 8 and a mass of ~16 amu. This indicates that there are 16 - 8 = 8 neutrons in the nucleus of an oxygen atom.
The number of electrons in an atom is always equal to the number of protons so long as the atom is neutral. When the number of protons (+ Charges) don't equal the number of electrons (- Charges) the atom is called an ion. Negatively charged atoms are called anions and positively charged atoms are called cations.
Ions form to increase the stability of the atom. Group VIII elements, the noble gases, are the most stable elements and have eight valence electrons (outermost shell electrons). All of the other elements in Groups I -VII form ions and bonds in an effort to obtain eight electrons in their outermost shell.
Example: Nitrogen is a Group V element. In order to become like the noble gas Neon, it must gain 3 electrons. Thus when Nitrogen forms ions, they have a 3- charge and when it forms bonds it generally bonds to three other elements.
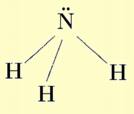
The structure of Ammonia shown above is a Lewis Structure. Lewis structures are representations of molecules that use lines for bonds and show dots for lone pairs of electrons. There are some basic rules for drawing Lewis structures that you should be familiar with:
Lewis structures are constructed in order to satisfy the octet rule for each of the atoms in a molecule. Bonds are represented by “-” and lone pairs of electrons are represented by “:”.
There are simple steps to creating a correct Lewis structure:
1)Calculate the total number of valence electrons available.2)Determine which atom will be central in the molecule.3)Arrange atoms symmetrically around the central atom.4)Place bonds/electrons around the atoms until the octet rule is satisfied for each atom. Use double or triple bonds if necessary.5)Show any charges on the molecule using brackets [ ] and place the charge in the upper right hand corner just outside the brackets
Step1: Calculate the total number of valence electrons available.
Let’s use PO43- as our example.
We need to know how many electrons are available to make the bonds for Phosphate Ion.
Phosphorus is in group VA so it has 5 valence electrons and Oxygen is in group VIA so each oxygen has 6 valence electrons. Total valence electrons = 5 + 4(6) = 29. BUT wait a minute, that is an odd number of electrons and we haven't really discussed that issue so something must be missing....Oh, the charge. For each negative charge on an ion, we need to add 1 valence electron so this makes the total 29 + 3 = 32 valence electrons.
This means we have 32 electrons to distribute into bonds to create phosphate ion.
Step 2: Determine which atom will be central in the molecule.
The central atom in a molecule is usually the least electronegative atom. It is also often the atom which will allow you to create the most symmetrical molecule. For phosphate, PO43-, the phosphate is the least electronegative atom and it will allow us to make the most symmetrical molecule so it is the most likely central atom. On a side note, for other molecules that contain hydrogens, we know that hydrogen can only make 1 bond so hydrogen atoms can NEVER be the central atom.
Step 3: Arrange atoms symmetrically around the central atom.
Most of nature’s creations are symmetrical and the same holds true for most chemical compounds. When writing Lewis structures, the most symmetrical arrangement of atoms around the central atom is best.
For phosphate: 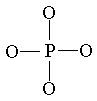 The four oxygen atoms are placed symmetrically around the phosphorus.
The four oxygen atoms are placed symmetrically around the phosphorus.
Step 4: Place bonds/electrons around the atoms until the octet rule is satisfied for each atom. Use double or triple bonds if necessary. You should use up all of the valence electrons. (Note the charge shown are formal charges on those ions)
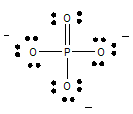
Step 5: Show any charges on the molecule using brackets [ ] and place the charge in the upper right hand corner just outside the brackets
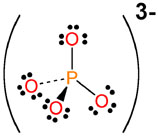
The Phosphate ion shown above can be combined with positive ions to form salts. A salt is an ionic compound (metal + non-metal) that is formed when two oppositely charged ions come together to form a neutral compound.
Example: Sodium Phosphate = Na3PO4 This is a salt composed of the Na+ and PO43- ions. At this point you should be able to construct the correct salt from any combination of positive and negative ions and name the resulting compound. If you still can't do this very well you should practice!!
Ionic compunds are considered the most polar form of molecule because they all dissociate in water to some extent. Other molecules, covalent molecules, do not dissociate into parts in water and are therefore considered non-polar.
A third group of molecules are both covalent and polar and therefore do mix to some degree with water. A great deal of the material in the first few chapters of CHM1046 will depend on your knowledge of these types of compounds so you will need to be able to discern whether a molecule is polar or not.
What causes polarity?
Polar molecules contain an electronegative atom that pulls the electrons in the molecule towards itself and away from the other atoms in the molecule. You can think of it as a popularity contest and the most electronegative atom is the most popular. The electrons all want to spend more time around it. When this happens the distribution of electrons (- Charges) are concentrated at one point in the molecule and the protons (+ Charges) of the other atoms are left somewhat exposed. This uneven distribution produces what is called a dipole and molecules that contain dipoles are considered to be polar.
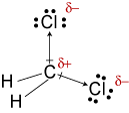 In this case, the Cl atoms are more electronegative than the carbon forming partial charges on the atoms and a dipole.
In this case, the Cl atoms are more electronegative than the carbon forming partial charges on the atoms and a dipole.
So which elements are electronegative? The most electronegative element is Fluorine and the trend of electronegativity increases from left to right and from bottom to top in the periodic table.
Other basic trends that you should be aware of are trends in ionization energy and atomic radius.
The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral atom. It generally increases from bottom to top (it is easier to take an electron away from an atom with lots of electrons than from one with just a few electrons) and from left to right in the periodic table. As you move from left to right across a period of the periodic table, you increase the number of protons in the nucleus but you remain within the same orbital shell. This means that the pull towards the nucleus is increasing as you go from left to right but the shielding (electrons in the inner shells) is remaining constant. If the electrons are being held onto tighter then the energy to break them free is going to increase. That is why elements on the far right of the table (except for the noble gases) all accept electrons far better than they give them up.
The atomic radius increases from top to bottom and from right to left in the periodic table. From top to bottom makes sense because you are adding large numbers of electrons, but the increase in size from right to left is often confusing. As you move from left to right across a period of the periodic table, you increase the number of protons in the nucleus but you remain within the same orbital shell. This means that the pull towards the nucleus is increasing as you go from left to right but the shielding (electrons in the inner shells) is remaining constant. This means that the electrons in the outer shell are being pulled in tighter and tighter as you go across the period and thus the atomic radius is shrinking.