New rules for alkyne cyclizations
Since up to 90% of organic molecules in nature contain either a carbocyclic or a heterocyclic subunit, it is not surprising that the 1976 paper on “Rules for Ring Closure” by Sir Jack E. Baldwin (Baldwin, J. E. J. Chem. Soc. Chem. Commun. 1976, 734) became the most cited article in the >40-year history of RSC Chemical Communications. One of the most remarkable conclusions from this classic work was that the rules for cyclizations of alkenes and alkynes are opposite: while alkenes (“trig”-cyclizations) the so-called “exo” path, alkynes (“dig”-cyclizations) favor the opposite “endo” selectivity.
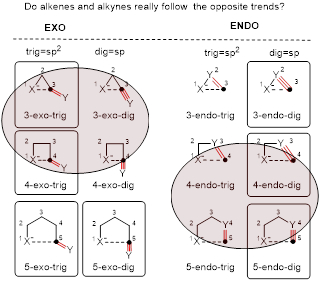
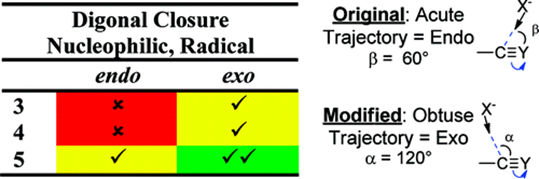
We have critically reevaluated the stereoelectronic basis for the “favored attack trajectories” that served as the basis for the cyclization rules. In contrast to the original Baldwin rules, the acute attack angle of a nucleophile leading to the proposed endo-dig preference for the formation of small cycles is less favorable stereoelectronically than the alternative obtuse trajectory leading to the formation of exo-dig products. The new experimental and theoretical data confirm that the earlier predicted rules for for alkyne cyclizations should be rewritten. The new rules are shown below. The rules are not absolute and we have also illustrated several possible ways of breaking them when an alternative selectivity is desired.
Selected publications:
Gilmore, K.; Alabugin, I. V. Cyclizations of Alkynes: Revisiting Baldwin’s Rules for Ring Closure. Chem. Rev. 2011, 111, 6513–6556. http://pubs.acs.org/doi/pdf/10.1021/cr200164y.
Alabugin, I. Gilmore, K.; Manoharan, M. Rules for Anionic and Radical Ring Closure of Alkynes. J. Am. Chem. Soc. 2011, 133, 12608-12623, http://pubs.acs.org/doi/abs/10.1021/ja203191f.
Finding the right path: Baldwin “Rules for Ring Closure” and stereoelectronic control of cyclizations. (Invited “Viewpoint”). Alabugin, I. V.; Gilmore, K. Chem. Commun., 2013, 49, 11246 – 11250. http://pubs.rsc.org/en/content/articlehtml/2013/cc/c3cc43872d.
Experimental studies aimed at testing the new rules and applying them for the design of new reactions:
Byers, P.; Alabugin, I. V. Polyaromatic Ribbons from Oligo-Alkynes via Selective Radical Cascade: Stitching Aromatic Rings with Polyacetylene Bridges, J. Am. Chem. Soc. 2012, 134, 9609–9614. http://pubs.acs.org/doi/abs/10.1021/ja3023626. (Highlighted in SYNFACTS)
Traceless Directing Groups in Radical Cascades: From Oligoalkynes to Fused Helicenes without Tethered Initiators. Pati, K.; Gomes, G. P.; Harris, T.; Hughes, A.; Phan, H.; Banerjee, T.; Hanson, K.; Alabugin, I. V. J. Am. Chem. Soc. 2015, 137, 1165-1180. http://dx.doi.org/10.1021/ja510563d.
Alkenes as Alkyne Equivalents in Radical Cascades Terminated by Fragmentations: Overcoming Stereoelectronic Restrictions on Ring Expansions For the Preparation of Expanded Polyaromatics. Mohamed, R.; Mondal, S.; Gold, B.; Evoniuk, C. J.; Banerjee, T.; Hanson, K.; Alabugin, I. V. J. Am. Chem. Soc., 2015, 137, 6335-6349. http://pubs.acs.org/doi/abs/10.1021/jacs.5b02373.