Stereoelectronically assisted biorthogonal chemistry (GEOSET presentation by Brian Gold)
The applications of “click chemistry” range from drug design and chemical biology to materials science, development of sensors, polymer chemistry and other molecular sciences. Although the copper-catalyzed variant of the Huisgen azide-alkyne cycloaddition (CuAAC) is arguably the most widely utilized “click reaction”, the toxicity of copper salts limits the utility of this fast and versatile process for in vivo applications.
We have identified two new strategies for selective transition state (TS) stabilization in catalyst-free azide/alkyne cycloadditions. The Scheme below shows how one can combine the two activating effects (hyperconjugative assistance and C-H…F interactions) to remove 80% of difference between butyne and cyclooctyne.
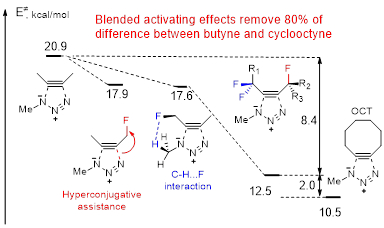
Combination of electronic activation with strain identifies alkynes that should be more stable and more reactive than cyclooctyne.
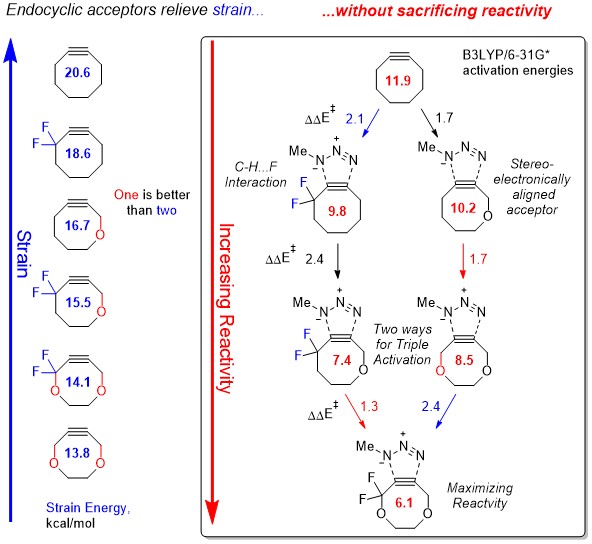
The goal of this research project (collaboration with Greg Dudley) is to experimentally verify these computational predictions and develop a new set of tools for biorthogonal chemistry.
Selected publications:
Alkynyl Crown Ethers as a Scaffold for Hyperconjugative Assistance in Non-catalyzed Azide-Alkyne Click Reactions: Ion Sensing through Enhanced Transition State Stabilization. Gold, B.; Batsomboon, P.; Dudley, G. B.; Alabugin, I. V. J. Org. Chem., 2014, 79, 6221–6232. http://pubs.acs.org/doi/full/10.1021/jo500958n.
Gold, B.; Dudley, G. B.; Alabugin, I. V. Moderating strain without sacrificing reactivity: Design of fast and tunable non-catalyzed alkyne-azide cycloadditions via stereoelectronically controlled transition state stabilization. J. Amer. Chem. Soc., 2013, 135, 1558-1569. http://pubs.acs.org/doi/abs/10.1021/ja3114196.
Gold, B.; Shevchenko, N.; Bonus, N.; Dudley, G. B.; Alabugin, I. V. Selective Transition State Stabilization via Hyperconjugative and Conjugative Assistance: Stereoelectronic Concept for Copper-Free Click Chemistry. J. Org. Chem. 2012, 77, 75−89. http://pubs.acs.org/doi/abs/10.1021/jo201434w.