Stereoelectronic effects in organic structure and reactivity
How adequate are the undergraduate organic foundations for the broad understanding of structure and reactivity? Do we really need to go deeper and continue to refine our knowledge?
We are interested in gaining better understanding of stereoelectronic effects, the ubiquitous family of phenomena that control interactions between different atoms or molecules and interactions between different parts of a single molecule.
In order to understand the importance of such effects, take a look at the following figure.
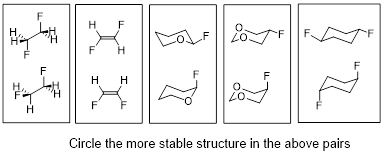
For each pair in the figure, the bottom structure is more stable than the top structure. The answer in each case is opposite to expectations based on the steric repulsion, a model that have served reasonably well as a foundation of undergraduate organic chemistry. Stereoelectronic interactions can provide guidance in understanding many surprising facts related to chemical structure and reactivity.
Selected publications:
Gabriel dos Passos Gomes, Vera Vil', Alexander Terent'ev, Alabugin, I. V. "Stereoelectronic source of the anomalous stability of bis-peroxides", Chem. Sci. 2015, in print. http://pubs.rsc.org/en/content/articlepdf/2015/sc/c5sc02402a.
Peterson, P. W.; Shevchenko, N.; Alabugin, I. V. “Stereoelectronic Umpolung”: Converting a p-Donor into a σ-Acceptor via Electron Injection and a Conformational Change. Org. Lett. 2013, 15, 2238. http://pubs.acs.org/articlesonrequest/AOR-3EcXR7Src3nze8eT72kg.
Alabugin, I. V.; Gilmore, K.; Peterson, P. Hyperconjugation. WIREs Comput Mol Sci 2011, 1, 109–141. http://onlinelibrary.wiley.com/doi/10.1002/wcms.6/abstract.
Gold, B.; Shevchenko, N.; Bonus, N.; Dudley, G. B.; Alabugin, I. V. Selective Transition State Stabilization via Hyperconjugative and Conjugative Assistance: Stereoelectronic Concept for Copper-Free Click Chemistry. J. Org. Chem. 2012, 77, 75−89. http://pubs.acs.org/doi/abs/10.1021/jo201434w.
Alabugin I. V.; Manoharan, M. “Effect of Double Hyperconjugation on the Apparent Donor Ability of σ-Bonds: Insights from the Relative Stability of δ-Substituted Cyclohexyl Cations”, J. Org. Chem. 2004, 69, 9011-9024.
Alabugin I.V.; Manoharan, M.; Zeidan, T. A. “Homoanomeric Effects in Saturated Heterocycles” J. Am. Chem. Soc. 2003, 125, 14014-14031.
Alabugin I.V.; Manoharan, M.; Peabody S.; Weinhold, F. “The Electronic Basis of Improper Hydrogen Bonding: A Subtle Balance of Hyperconjugation and Rehybridization.” J. Am. Chem. Soc. 2003, 125, 5973-5987.
Alabugin I. V.; Zeidan, T. A. “Stereoelectronic Effects and General Trends in Hyperconjugative Acceptor Ability of σ Bonds.” J. Am. Chem. Soc. 2002, 124, 3175-3185.