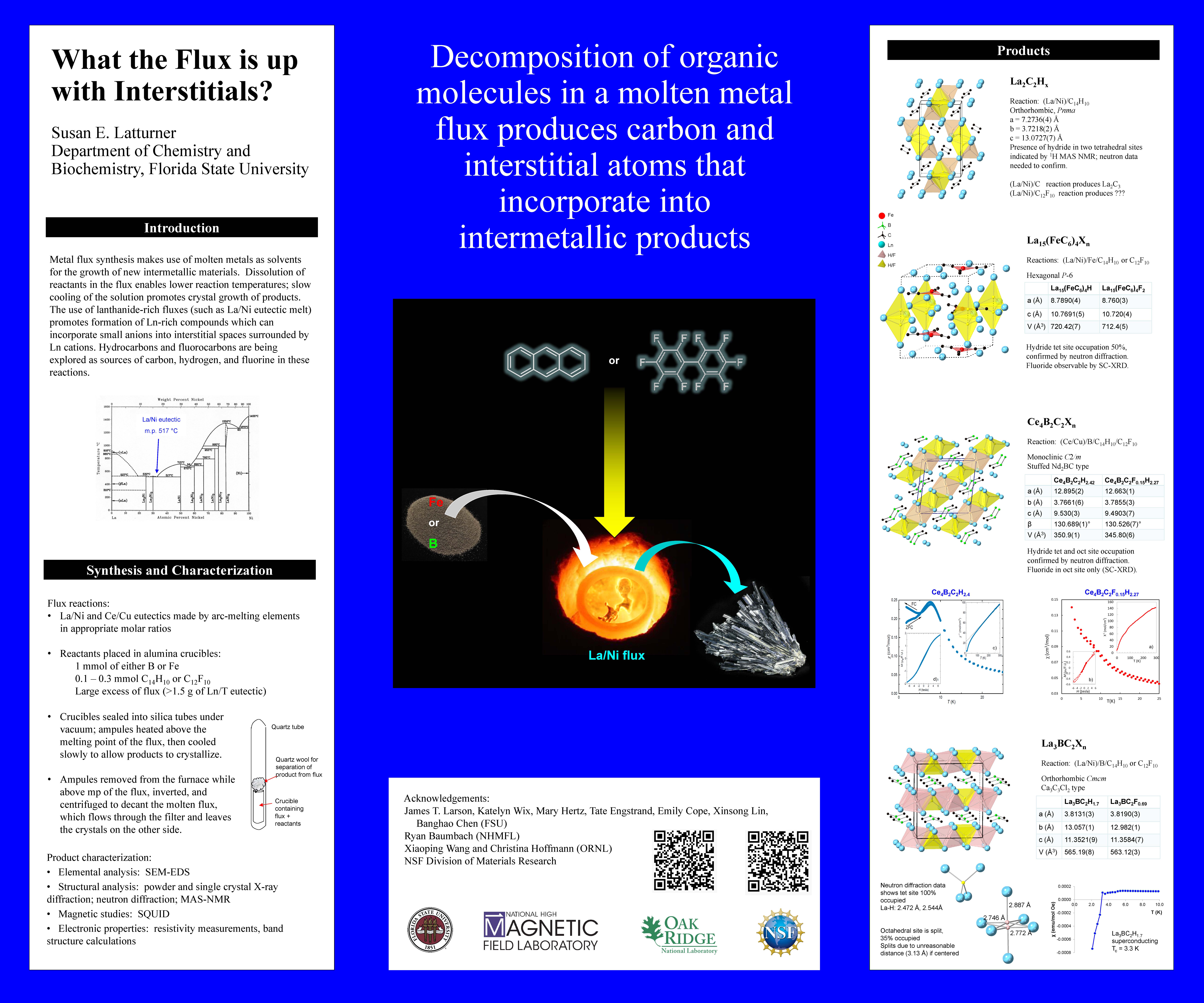
 Reactions of Fe, Si, and C in an excess of Pr/Ni flux produce two competing phases with common structural features.
Reactions of Fe, Si, and C in an excess of Pr/Ni flux produce two competing phases with common structural features. Research
Metal flux growth of new intermetallics
Molten metals have been used as solvents for the growth of a variety of compounds, ranging from large single crystals of silicon to exploratory synthesis of new intermetallic materials. Reactions in metal fluxes can be carried out at lower temperatures than those typically required for traditional solid state synthesis, allowing for the formation of metastable or kinetically stabilized products. Flux reactions are also solution-state reactions, allowing for crystal growth with slow cooling of the melt.
Low melting metals such as gallium, indium, and tin have been well-explored as fluxes. We are investigating combinations of different metals to create unique reaction solvents for new materials. In many cases, combining two metals at specific ratios results in a lowered melting point (for instance, eutectic formation); this enables high-melting metals that have not been previously considered as fluxes to be used. Our investigation into metal flux reactions includes the production of new intermetallic phases with interesting electronic and magnetic behavior or with potential use as optical or thermoelectric materials. This research is funded by the National Science Foundation.
--Magnetic intermetallics from Ln/T eutectics
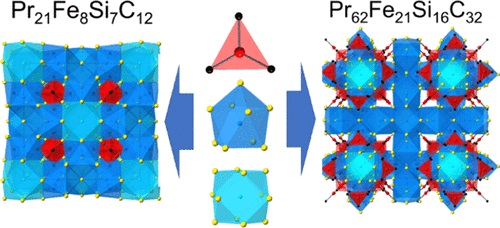
The hard magnetic materials used in computer hard drives are intermetallics such as SmCo5 and Nd2Fe14B. Their strong ferromagnetism is derived from the coupling of the local moments of the unpaired electrons on the lanthanide ion with the itinerant magnetism of the transition metal valence electrons. The binary phase diagrams of early lanthanides (Ln = La, Ce, Pr) combined with late transition metals (Ni, Fe) all feature low melting eutectics in the lanthanide-rich region which may act as fruitful synthesis media for magnetic compounds containing two paramagnetic elements. We have reported on the synthesis of new materials containing clusters and layers of iron separated by lanthanum ions from La/Ni eutectic (melting point 532 C). Even more complex phases are now being formed in reactions of metals and metalloids in Ce/Co and Pr/Co eutectic fluxes (melting points 424 and 541 C, respectively)! Structural building blocks that are commonly observed are FeC3 trigonal planar units and heavy main group atoms surrounded by 9 - 12 lanthanide ions. These building blocks can assemble different ways to yield competing products. The magnetic properties of these phases are characterized using techniques such as temperature and field dependent magnetic susceptibility measurements, Mossbauer spectroscopy, and neutron diffraction.
 Reactions of Fe, Si, and C in an excess of Pr/Ni flux produce two competing phases with common structural features.
Reactions of Fe, Si, and C in an excess of Pr/Ni flux produce two competing phases with common structural features.
--Complex semimetallic tetrelides from alkaline-earth based fluxes
We have found that magnesium-based fluxes are excellent reaction media for the growth of complex tetrelides (Tt = Si, Ge, Sn). Reactions of heavy divalent metals (A = Ca, Sr, Ba, Eu, Yb) with silicon in Mg/Al melts produce a variety of AxMgySiz semimetals. Compounds such as CaMgSi, Eu5Mg19Si12, and Ba2Ca2Mg10Si7 can be grown as large crystals, facilitating the study of their electronic properties. Their complex structures, substitutional chemistry, and semi-metallic behavior make them potential thermoelectric materials.
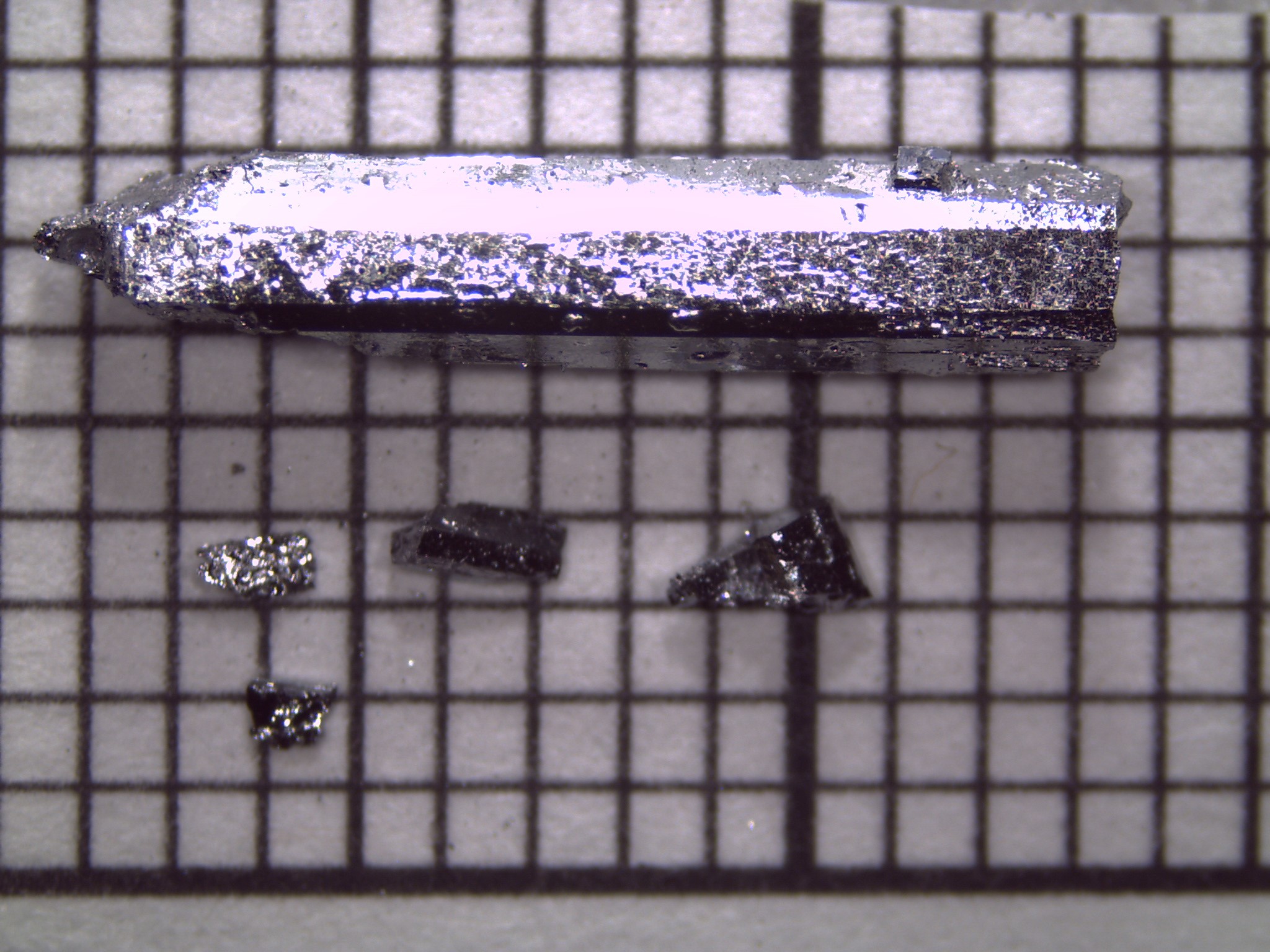 Flux-grown crystals of Ba2Ca2Mg10Si7 grown from Mg/Al flux.
Flux-grown crystals of Ba2Ca2Mg10Si7 grown from Mg/Al flux.
--Incorporation of interstitial atoms into intermetallics during flux growth
Thermal decomposition of organic molecules above a lanthanide-rich melt yields carbon and hydrogen, which are incorporated into the growing intermetallic compounds. Several new lanthanide carbide hydrides have been produced. The addition of hydride ions can lead to changes in electronic and magnetic properties. We have now expanded this method to making compounds with fluoride interstitials.