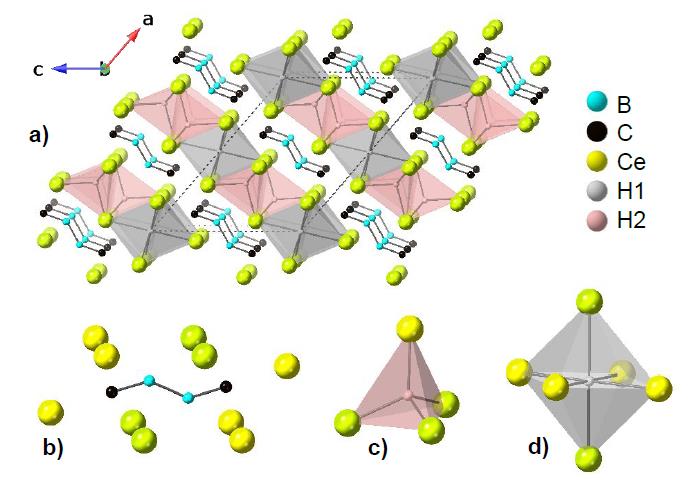
Ce4B2C2H2.42
This unexpectedly hydrided phase was first grown from the reaction of carbon and boron in an excess of cerium/copper eutectic melt. The cerium likely contained some CeH2 or surface hydroxides that acted as the hydride source. Subsequent reactions using anthracene as a carbon source increased the yield of product. The structure contains borocarbide chains in addition to hydride sites in octahedral and tetrahedral interstitial sites, confirmed by neutron diffraction. This is described in our manuscript submitted to Crystal Growth and Design.
