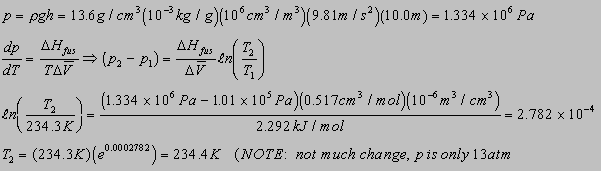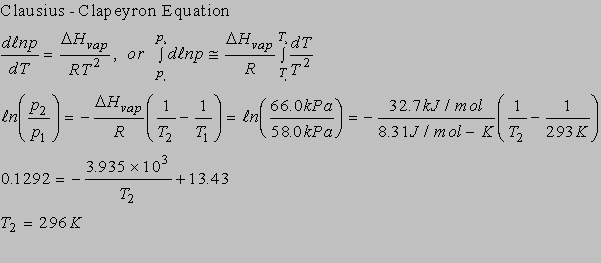
6.2(b)
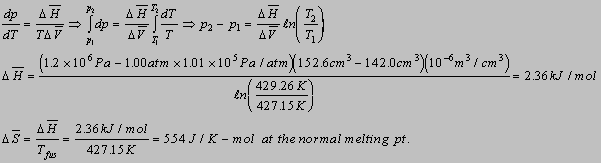
Chapter 6, Exercises #6.1(b), 6.2(b), 6.3(b), 6.4(b), 6.5(b), 6.7(b), 6.8(b), 6.10(b); Problems #6.1, 6.2, 6.6
6.1(b)
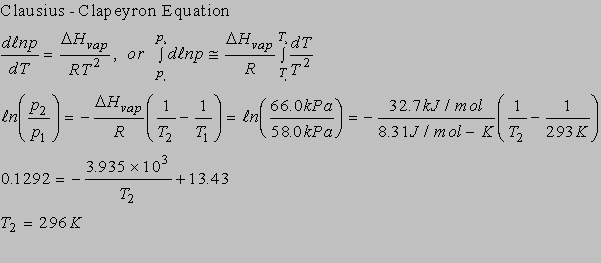
6.2(b)
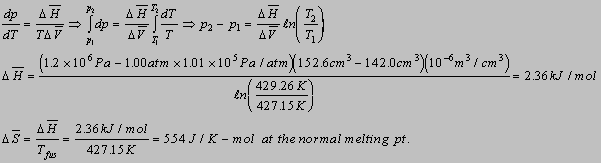
6.3(b)
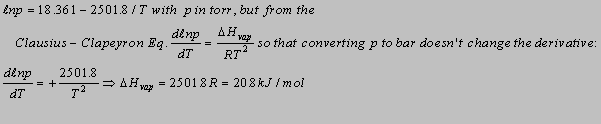
6.4(b)
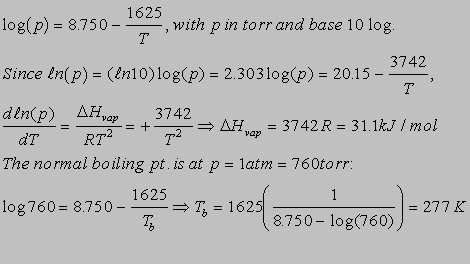
6.5(b)
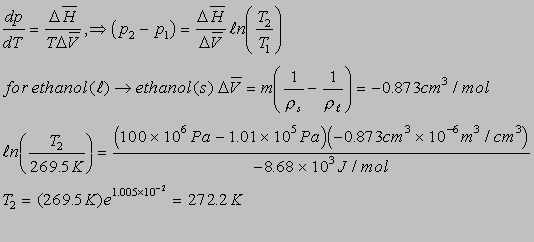
6.7(b)
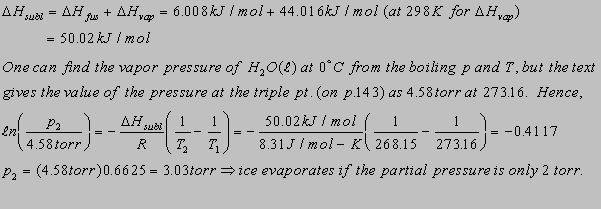
6.8(b)
a) At 1 atm, 298K CO2 is a gas. For isobaric heating to 320K, the gas expands in volume to satisfy equation of state and is still a gas.
b) When it is compressed to 100 atm isothermally, no liquid phase is formed since it is beyond the critical temperature of 304.2K. The gas just gets denser and denser as it is compressed.
c) When it is cooled isobarically to 210K, it goes into a solid phase along the path.
d) When it is isothermally decompressed to 1 atm, it goes directly into the gas phase again (i.e., no liquid phase) since the triple point is at a higher temperature and pressure, 216.8K at 5.11atm and it is also above the normal boiling temperature 194.7K.
e) Isobaric heating to 298K brings it back to its initial state, which is still the in the gas phase.
6.10(b)
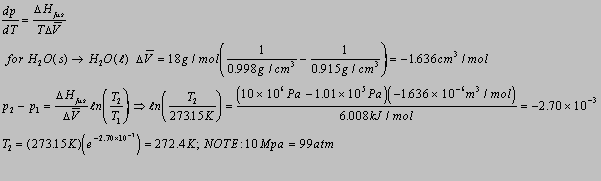
6.1
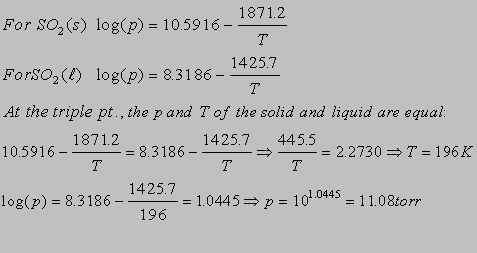
6.2
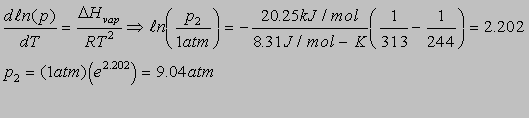
6.6