Homework# 10
November 18, 1998
Due: Monday, November 23
1. (a) Calculate the standard cell potential at 298 K for the reaction
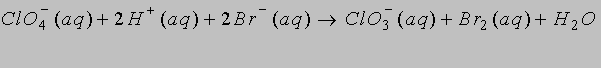
from the standard reduction potentials in Table 10.7. (Write out the half-cell reactions.)
(b) Write an expression for the reaction quotient Q for this reaction in terms of the activities and then in terms of the concentrations of each of the species in the reaction.
(c) Find the equilibrium constant for this reaction at 298 K.
2. Find the equilibrium constant at 298 K for the solubility of AgCl in water, i.e., the process
![]()
by using the reduction potentials from Table 10.7 for the reduction
of AgCl to Ag and the reduction of Ag+ to Ag.
3. (a) Write out the half-cell reactions for a fuel cell which "combusts" methane, for (i) acidic and (ii) basic solutions.
(b) From the Gibbs free energy of combustion of methane and reduction potentials in Table 10.7, find the half-cell and overall cell potentials at 298 K for each half-reaction and overall reaction in part (a).
(c) Does the cell potential for the fuel cell increase or decrease as T increases? Explain briefly.
4. Batteries are rated by their "ampere-hours", the total amount of charge they can deliver. How much methane (in grams) must be consumed in the fuel cell of problem (3) for 1 ampere-hour?