Heterogeneous Catalysis
Intermetallic catalysts for water splitting and petroleum reforming
In this project, we investigate layered intermetallics (LIMs) and complex metallic alloys (CMAs) as catalysts in water splitting and petroleum reforming processes. Heuristic approaches fail to predict catalytic activity of such complex structures. To combat this issue, we employ data mining, machine learning, and DFT calculations to analyze the vast range of known intermetallic crystal structures and discover LIMs and CMAs that can exhibit catalytic activity with respect to the reactions of interest.
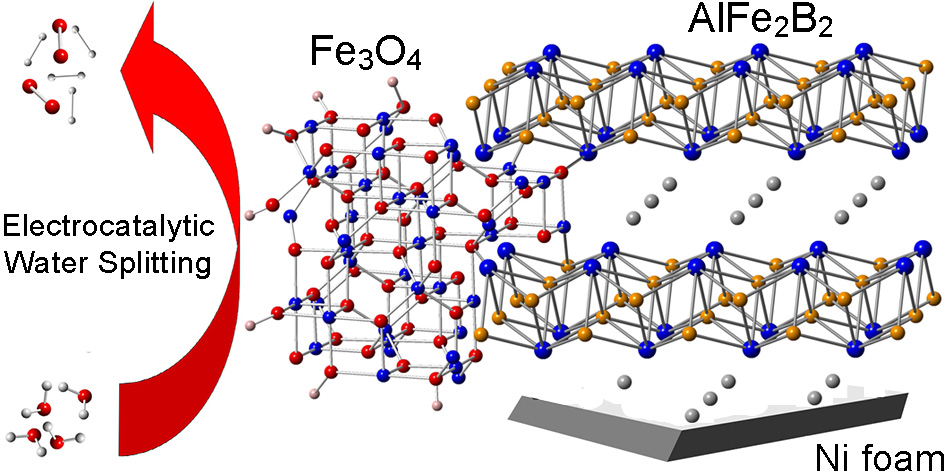
Figure 1. Oxidation of the Fe-sites in AlFe2B2 provides catalytically active sites.
Complex Metallic Alloys
Complex metallic alloys (CMAs) have been long viewed as crystallographic curiosity rather than as applied materials.1 The situation has been changing over the last decade, as the advanced imaging instrumentation and more powerful computational techniques afforded deeper insight into the structure-property relationships of CMAs. These materials are beyond the realm of 3-D periodic crystal structures. They are characterized by large unit cells which often cannot be adequately described in the conventional 3-D space. An extreme example is offered by quasicrystals, the discovery of which by Shechtman2 earned him the 2011 Nobel prize in chemistry and dramatically shifted our view of what the term “crystal structure” might mean.
Layered Intermetallics
Layered intermetallics (LIMs) have been used recently as effective water splitting catalysts by us3 and other research groups. We view them as ideally structured materials for applications in heterogenous catalysis in many technologicallly important processes.
Researchers involved in this project gain skills in the synthesis of intermetallic compounds, X-ray diffraction analysis of bulk and nanoscale materials, surface characterization by scanning electron microscopy and X-ray photoelectron spectroscopy, electrochemistry and electrocatalysis, and band structure calculations at the density-functional level of theory.
References
- 1. DuBois, J.-M. Useful Quasicrystals. World Scientific: Singapore, 2005; 504 pp.
- 2. Shechtman, D.; Blech, I.; Gratias, D.; Cahn, J. W. Phys. Rev. Lett.1984, 53, 1951-1953.
- 3. Mann, D.; Xu, J.; Mordvinova, N.; Yanello, V.; Ziouani, Y.; Gonzalez-Ballesteros, N.; Sousa, J.; Lebedev, O.; Kolen'ko, Y.; Shatruk, M. Chem. Sci. 2019, 10, 2796-2804.

