What you need to do:
Procedure: Precipitating Cu2+ ions as copper metal
WARNING: The copper sulfate solution contains concentrated hydrochloric acid that is corrosive and can cause severe burns. Handle with care. Dilute and wash up spills with plenty of water. Wash your hands immediately if you come in contact with the solution.
- Collect and weigh all of the following items and record the masses in your notebook to the nearest 0.0001 g.
- A 100 mL side-arm flask
- 5 or 6 pieces of solid zinc metal (weigh together on a clean Kimwipe)
- 1 Kimwipe
2. Place 30 mL of the copper sulfate solution in the sidearm flask and re-weigh. Record the mass in your lab notebook to the nearest 0.0001g.
3. Once all of the masses are recorded, fit the balloon onto the sidearm of the pre-weighed flask. Using a rubber band or parafilm to make sure the seal of the balloon to the sidearm is airtight.
4. Collect a stopper from the front counter.
5. You are now ready to start the reaction. Quickly place the pre-weighed zinc metal pieces into the solution and cap with the stopper. You should see bubbles form right away. This is the hydrogen gas being produced.
6. Make observations about how the solution’s appearance changes as the reaction proceeds, noting if there are any color changes. Make sure the balloon stays on the sidearm of the flask as increased gas pressure might push it off.
7. When the reaction is complete (no more bubbles and the solution should be clear) you will need to carefully remove the balloon trapping as much gas inside it as possible. This is done by pinching the lip of the balloon, just beyond the end of the sidearm and then carefully pulling it free. Once you have the balloon free of the flask you can seal the gas inside by tying a knot in the neck of the balloon.
8. Once you have tied the balloon off, measure the radius and calculate the volume by using
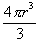 = Volume of a spherical balloon = Volume of a spherical balloon
or
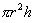 = Volume of a cylindrical balloon. = Volume of a cylindrical balloon.
We will calculate the mass of hydrogen gas by using the following equation (this is part of what is called the ideal gas law). Assuming the temperature of the gas is approximately room temperature, 298 K (or 25 oC) and that the atmospheric pressure is 1.000 atmospheres, you can calculate the mass of hydrogen gas by inserting the volume of hydrogen gas in L into the following equation:
Mass of H2 gas (in g) evolved = 8.244 X 10-2 x (volume in L)
[Reminder: 1 cm3 = 1 mL = 0.001 L]
9. We now need to measure both the metals and solution that remain in the flask. First you will need to remove all of the metal pieces from the solution. Do this by using the tongs/tweezers in your drawer. Carefully pull each metal piece out, allow any excess liquid to fall back into the solution in the flask. Place the metal pieces on a paper towel to dry.
10. Weigh the solution that remains in the flask and record the weight in your notebook to the nearest 0.0001g.
11. Once dry, place the metal pieces on a clean Kimwipe and weigh them. Record the weight in your notebook to the nearest 0.0001g.
|

