|
Experiment 5 Polarity and Solubility: Halogen Reactions
|
Introduction/Background

Dimitri Mendeleev | |
Dimitri Mendeleev (1834- 1907) invented the first periodic table. He arranged the elements by their increasing atomic masses. Elements with similar properties were put one below the other, in columns. He even left blanks in the table because he figured there would be other elements to fit the missing properties.
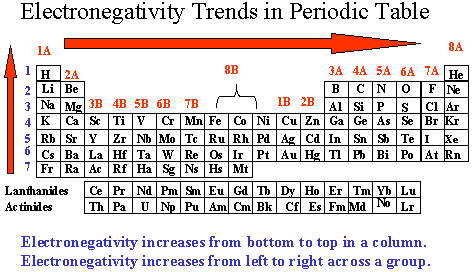
The second periodic table was formatted by a British physicist named Henry Moseley (1887- 1915). He discovered a nuclear charge and called it atomic number and arranged the periodic table by their atomic numbers, which is like the periodic table now. Today's periodic table is arranged in seven rows called periods (arranged in increasing atomic number). The vertical columns are called groups. Types of groups are: Alkali Metals, Alkaline Earth Metals, Transition Metals, Chalcogens, Halogens, and Noble Gases. Your lab today will focus on properties of the Halogens. |
| The periodic table is strategically put together so that trends of the elements are easily defined. This lab focuses on the trend of electronegativity (specifically electronegativity of the halogens), which increases as you go up a vertical group in the periodic table. Elements near the top of the periodic table have fewer electrons to begin with. Therefore, every electron is a big deal. The elements on the right at the top have a stronger desire to gain more electrons. Elements near the bottom of the chart have so many electrons that loosing or gaining an electron is not as big a deal. In order to prove that the trend for electronegativity increases as you go up the halogen column, we will be utilizing solubility properties of the halogens in hexane (a non-polar solvent). Remember: example of halogen = Cl2 example of halide = Cl- . |

Henry Moseley |
| NOTE: Halogens are four of seven common diatomic elements. These are F2, Cl2, Br2, and I2. Halides are the ionic versions of the halogens (meaning they have gained an electron to become like the noble gases). These include F-, Cl-, Br-, and I-. |
Key Concepts
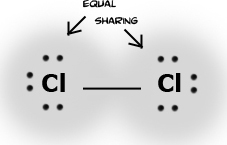
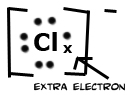
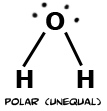
Electronegativity is the power of an atom when in a molecule to attract electrons to it. Electronegativity is a function of the atom's ionization energy and the atom's electron affinity. An atom that has both high electron affinity and high ionization energy has the ability to attract electrons from other atoms and resist having its own electrons taken from it. Electrons shared between two atoms are not necessarily shared equally. In Cl2 electrons are shared equally. This bond is called a non-polar covalent bond. In water the oxygen atom is more electronegative than the hydrogens. Therefore, the electrons are not shared equally, and this type of bond is called polar covalent. Finally, table salt (NaCl) has a bond in which electrons are not shared at all. This bond is called ionic.
The polar substances are Cl- , Br- , I- , water; non-polar substances are Cl2, Br2, I2, hexane. Like dissolves like. Therefore, all the ionic substances will dissolve in water (and NOT in hexane), and all non-ionic/non-polar substances will dissolve in hexane (and NOT in water). Also, remember your electronegativity trends. Atomic chlorine is much more electronegative than atomic bromine, which is more electronegative than atomic iodine. So if Cl2 is combined with Br- , a reaction will occur because a chlorine atom wants electrons much more than bromide wants to keep its extra electron. Simple? Let's do some examples.
|
 |
Example 1: When Br2 is combine with I- , will a reaction occur? If so, what are the products? Br2 + I- →
Bromine atom is more electronegative than iodine atom, therefore, it will win in the fight for electrons, and the reaction will be: Br2 + 2I- → 2Br- + I2 |
Example 2: When I2 is combined with Cl- , will a reaction occur? If so, what are the products: I2 + Cl- →
Iodine atom is less electronegative than chlorine atom, and chlorine is very happy with its extra electron since it is an electronegative element. Therefore a reaction will not occur. I2 + Cl- → no reaction. |
In this lab you will be able to visually see whether or not these reactions will occur by using color changes. Cl2, Br2, and I2 are all colored elements. Cl- , Br- , I- , water, and hexane are all colorless. When water and hexane are combined, they form two distinct colorless layers, with the hexane sitting right on top of the water. If Cl2 is added to the water/hexane combo, the hexane layer should be colored. Why? Remember, like dissolves like. Let's do another example.
Example 3: If a orange Br2 solution is added to the water/ hexane combo, the hexane layer will turn orange. What happens if you now add Cl-
to the Br2/ hexane/ water?
Answer: click to reveal |
Example 4: If a yellow Cl2 solution is added to the water/ hexane combo, the hexane layer will turn yellow. What happens if you now add Br- to the Cl2/ hexane/ water?
Answer: click to reveal |
By combining all the different halogens with all of the different halides, you will be observing whether or not the color of the hexane layer changes. You will be able to confirm whether or not a reaction has occured.
Related Materials
For more information about the periodic table:
WebElements Periodic Table (http://www.webelements.com/index.html)
Chemicool Periodic Table (http://www-tech.mit.edu/Chemicool/)
For more information about electronegativity trends:
Periodic Table of the Elements Electronegativity (http://web.mit.edu/3.091/www/pt/pert8.html)