|
FSU publications Stereoelectronic Effects: the Bridge between Structure and Reactivity. Alabugin, I. V., John Wiley & Sons Ltd, 2016. (Book available from Wiley or Amazon)
Oxygen: the Key to Stereoelectronic Control in Chemistry. Alabugin, I. V. and Kuhn L. , ACS in Focus, 2023. (Book available from ACS)
Submitted: 209. Tethering Three Radical Cascades for Controlled Termination of Radical Alkyne peri-Annulations: Making Phenalenyl Ketones without Oxidants. C. Hu, L. Kuhn, F. D. Makurvet, E. S. Knorr, X. Lin, R. K. Kawade, K. Hanson, I. V. Alabugin, J. Amer. Chem. Soc., 2023, under revision. In print: 208. Bioinspired Fe(II)-mediated halogenative C-C bond activation of ozonides: Temporary installment of a peroxide bridge allows selective C-C scissions for replacement of a carbonyl group by a halogen. I. A. Yaremenko, Y. Y. Belyakova, A. A. Demina, P. S. Radulov, I. V. Alabugin, A. O. Terent’ev, Adv. Synth. & Catalysis, 2023,accepted . Published: 2023 207. An Unusual Rearrangement of Pyrazole Nitrene and Ring Opening/Recyclization Cascade: Formal CH-Acetoxylation and Azide/Amine Conversion without External Oxidants and Reductants. E. Gibadullina, M. Neganova, Y. Aleksandrova, N. H. B. Tran, A. Voloshina, M. Khrizanforov, N. T. Thu, E. Vinyukova, K. Volcho, D. Tsypyshev, A. Lyubina, S. Amerhanova, A. Strelnik, J. Voronina, D. Islamov, R. Zhapparbergenov, N. Appazov, B. Chabuka, K. Christopher, A. Burilov, N. Salakhutdinov, O. Sinyashin, I. Alabugin Molecules, 2023, 28,7335.
206. “Stereolectronic deprotection of nitrogen”: recovering nucleophilicity with a conformational change. A.S. Gazizov, A. V. Smolobochkin, T. S. Rizbayeva, S. Z. Vatsadze, A. R. Burilov, O. G. Sinyashin, I. V. Alabugin, J. Org. Chem., 2023, 88,6868–6877.
205. Photochemical Uncaging of Aldehydes and Ketones via Photocyclization/Fragmentation Cascades of Enyne Alcohols: An Unusual Application for a Cycloaromatization Process. A. Campbell, N. R. Dos Santos, I. V. Alabugin, Molecules, 2023, 24,(special issue for V. Ramamurthy).
204. Hybrids of Sterically Hindered Phenols and Diaryl Ureas: Synthesis, Switch from Antioxidant Activity to ROS Generation and Induction of Apoptosis. E. Gibadullina, M. Neganova, Y. Aleksandrova, N. H. B. Tran, A. Voloshina, M. Khrizanforov, N. T. Thu, E. Vinyukova, K. Volcho, D. Tsypyshev, A. Lyubina, S. Amerhanova, A. Strelnik, J. Voronina, D. Islamov, R. Zhapparbergenov, N. Appazov, B. Chabuka, K. Christopher, A. Burilov, N. Salakhutdinov, O. Sinyashin, I. Alabugin, Int. J. Mol. Sci. , 2023, 28,12637. 203. Anticancer and Antiphytopathogenic Activity of Fluorinated Isatins and Their Water-Soluble Hydrazone Derivatives. A. Bogdanov, M. Neganova, A. Voloshina, A. Lyubina, S. Amerhanova, I. Litvinov, O. Tsivileva, N. Akylbekov, R. Zhapparbergenov, Z. Valiullina, A. Samorodov, I. Alabugin, Int. J. Mol. Sci., 2023, 24,15119. 202. Two-component vs three-component condensations in the race between hydrazide, triketone and hydrogen peroxide – how do all six reactive centers cooperate to incorporate the most diverse set of heteroatomic bridges in a tricyclic frame?. I. A. Yaremenko, Y. Yu. Belyakova, P. S. Radulov, M. G. Medvedev, N. V. Krivoshchapov, I. V. Alabugin, A. O. Terent’ev, J. Org. Chem., 2023, 88,13782–13795.
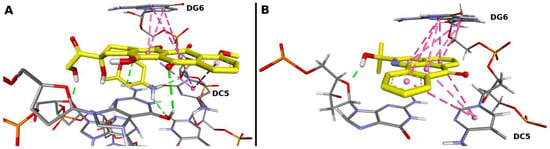
201. Hole Catalysis of Cycloadditions: How to Activate and Control Oxidant Upconversion in Radical-Cationic Diels-Alder Reactions. B. K. Chabuka and I. V. Alabugin, J. Am. Chem. Soc., 2023,145, 35, 19354–19367.
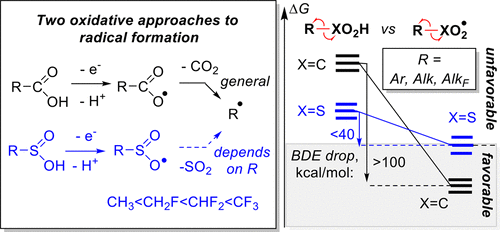
200. Creating, Preserving, and Directing Carboxylate Radicals in Ni-Catalyzed C(sp3)–H Acyloxylation of Ethers, Ketones, and Alkanes with Diacyl Peroxides. Vera A. Vil’, Yana A. Barsegyan, Leah Kuhn, Alexander O. Terent’ev, and Igor V. Alabugin, Organometallics, 2023, Special Issue dedicated to I. P. Beletskaya.
199. Diverse Biological Activity of Benzofuroxan/Sterically Hindered Phenols Hybrids. E. Chugunova, E. Gibadullina, K. Matylitsky, B. Bazarbayev, M. Neganova, K. Volcho, A. Rogachev, N. Akylbekov, H. Bao T. Nguyen, A. Voloshina, A. Lyubina, S. Amerhanova, V. Syakaev, A. Burilov, N. Appazov, M. Zhanakov, L. Kuhn, O. Sinyashin, I. Alabugin, Pharmaceuticals , 2023, 16, 4, 499 .
198. Design principles of the use of alkynes in radical cascades. C. Hu, J. Mena, I.V. Alabugin, Nature Reviews Chemistry, 2023
197. AIBN as an Electrophilic Reagent for Cyano Group Transfer. Quintin Elliott and Igor V. Alabugin, J. Org. Chem., 2023, 88, 4, 2648 - 2654.
2022 196. Design and Synthesis of Kekulè and Non-Kekulè Diradicaloids via the Radical Periannulation Strategy: The Power of Seven Clar’s Sextets. Febin Kuriakose, Michael Commodore, Chaowei Hu, Catherine J. Fabiano, Debashis Sen, Run R. Li, Shubham Bisht, Ökten Üngör, Xinsong Lin, Geoffrey F. Strouse, A. Eugene DePrince III, Robert A. Lazenby, Frederic Mentink-Vigier, Michael Shatruk, and Igor V. Alabugin. J. Am. Chem. Soc., 2022, 144, 51, 23448 - 23464.
195. The α-Methylstilbene Isomers – Relationship of Structure to Photophysics and Photochemistry. Krishnan, S.; Clark, R.; Lin, X.; Dmitrenko, O.; Hilinski, E.; Kuhn, L.; Alabugin, I.; Saltiel, J. J. Phys. Chem. A., 2022, 126, 48, 8976 - 8987.
194. Activation of O-Electrophiles via Structural and Solvent Effects: SN2@O Reaction of Cyclic Diacyl Peroxides with Enol Acetates. V. A. Vil, E. S. Gorlov, D. V. Shuingalieva, A. Yu. Kunitsyn, N. V. Krivoshchapov, M. G. Medvedev, I. V. Alabugin, A. O. Terentev. J. Org. Chem., 2022, 87, 13980 - 13989.
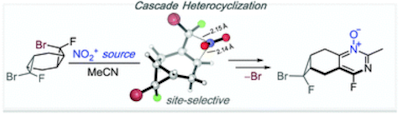
193. Electron upconversion in reactions of 1,2,4-triazoline-3,5-dione. V. A. Balycheva, A. Ya. Akyeva, E. A. Saverina, P. G. Shangin, I. V. Krylova, V. A. Korolev, M. P. Egorov, I. V. Alabugin, M. A. Syroeshkin. Russian Chemical Bulletin, 2022, 71, 1614 - 1625. 192. Cascade assembly of bridged N-substituted azaozonides: The counterintuitive role of nitrogen source nucleophilicity. I. A. Yaremenko, Y. Y. Belyakova, P. S. Radulov,R. A. Novikov, M. G. Medvedev, N. V. Krivoshchapov, I. V. Alabugin, A. O. Terent’ev. Org. Lett., 2022, 24, 6582 - 6587.
191. A Swiss Army knife for surface chemistry. I. V. Alabugin; C. Hu. Science, 2022, 377, 261 - 262.
190. Two Paths to Oxidative C-H Amination Under Basic Conditions: A Theoretical Case Study Reveals Hidden Opportunities Provided by Electron Upconversion. P. Eckhardt, Q. Elliott, I. V. Alabugin, T.Opatz. Chem. Eur. J., 2022, 28, e202201637.
189. Carboxylate as a non-innocent L-ligand – computational and experimental search for metal-bound carboxylate radicals. Kuhn, L.; Vil', V. A.; Barsegyan, Y. A.; Terent'ev, A. O.; Alabugin, I. V. Org. Lett., 2022, 24, 3817 - 3822.
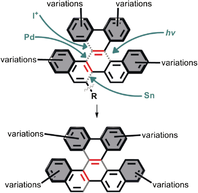
188. Localized Antiaromaticity Hot-spot Drives Reductive Dehydrogenative Cyclizations in Bis- and Mono-Helicenes. Zheng Zhou, Dominic T. Egger, Chaowei Hu, Matthew Pennachio, Zheng Wei, Rahul K. Kawade, Ökten Üngör, Renana Gershoni-Poranne, Marina A. Petrukhina, Igor V. Alabugin. J. Am. Chem. Soc., 2022, 144, 12321 - 12338.
187. Visible Light-driven Metal-free C–H Functionalization: Access to New Bioactive Tetrahydroisoquinoline-Butenolide Hybrids via Domino Amine Oxidation/Vinylogous Mannich Reaction. L. Kersting; L. Kuhn; M. Anokhin; F.Schuster; C. Häberli; S. Sambyal; H. M. S. Kumar; J. Keiser; I. V. Alabugin; S. B. Tsogoeva. ChemPhotoChem, 2022, e202200109.
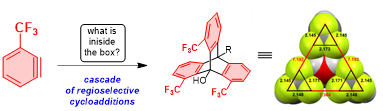
186. 3-Trifluoromethylbenzyne: Precise Orientation in Cycloaddition Reaction Enabled Regioselective Synthesis of Trifluoromethylated Triptycenes. Takayuki Iwata , Mizuki Hyodo , Takumi Fujiwara , Leah Kuhn , Igor V. Alabugin , Mitsuru Shindo. Synthesis, 2022
185. Remote Stereoelectronic Effects in Pyrrolidone- and Caprolactam-Substituted Phenols: Discrepancies in Antioxidant Properties Evaluated by Electrochemical Oxidation and H-Atom Transfer Reactivity. Anna Ya. Akyeva, Artem V. Kansuzyan, Katarina S. Vukich, Leah Kuhn, Evgeniya A. Saverina, Mikhail E. Minyaev, Valery M. Pechennikov, Mikhail P. Egorov, Igor V. Alabugin, Stepan V. Vorobyev, and Mikhail A. Syroeshkin. J. Org. Chem., 2022, 87, 5371 - 5384.
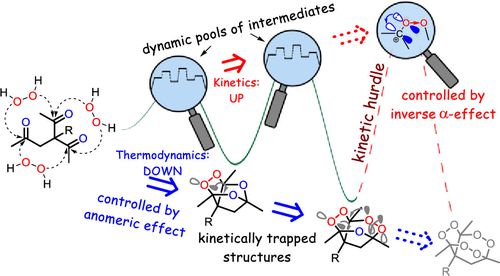
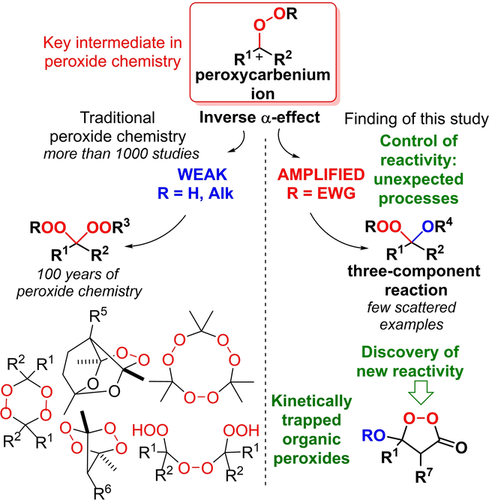
184. Inverse α-Effect as the Ariadne’s Thread on the Way to Tricyclic Aminoperoxides: Avoiding Thermodynamic Traps in the Labyrinth of Possibilities. I. A. Yaremenko, Y. Yu. Belyakova, P. S. Radulov, Roman A. Novikov, M. G. Medvedev, N. V. Krivoshchapov, A. A. Korlyukov, I. V. Alabugin, A. O. Terent’ev. J. Am. Chem. Soc., 2022, 144, 7264 - 7282.
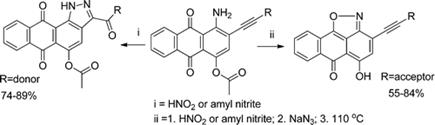
2021 183. New Heterocycles via an Intriguing Visible-Light-Promoted 5-endo-dig Cyclization. Alabugin, I. V.; Hu, C. Chem. Catalysis, 2021, 1, 976-975.
182. Cascade Transformations of 1-R-Ethynyl-9,10-anthraquinones with Amidines: Expanding Access to Isoaporphinoid Alkaloids. S. F. Vasilevsky, O.L. Krivenko, I. V. Sorokina, D. Baev, T. G. Tolstikova, I. V. Alabugin. Molecules, 2021, 26, 6883-6896.
181. Expanding Stereoelectronic Limits of endo-tet Cyclizations: Synthesis of Benz[b]azepines from Donor–Acceptor Cyclopropanes. A. E. Vartanova, A. Yu. Plodukhin, N. K. Ratmanova, I. A. Andreev, M. N. Anisimov, N. B. Gudimchuk, V. B. Rybakov, I. I. Levina, O. A. Ivanova, I. V. Trushkov, I. V. J. Am. Chem. Soc., 2021, 143, 13952-13961.
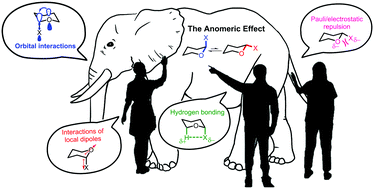
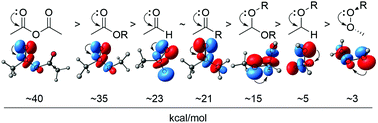
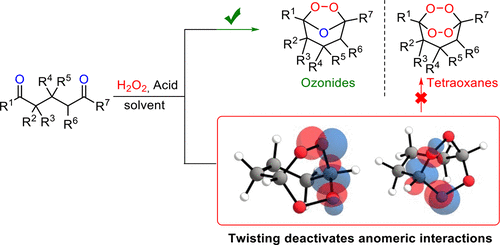
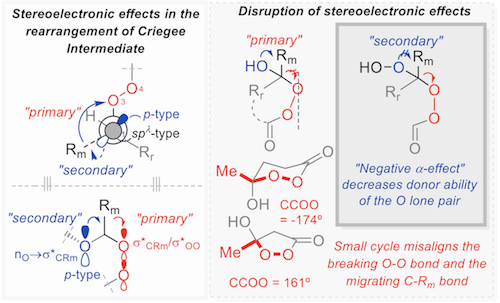
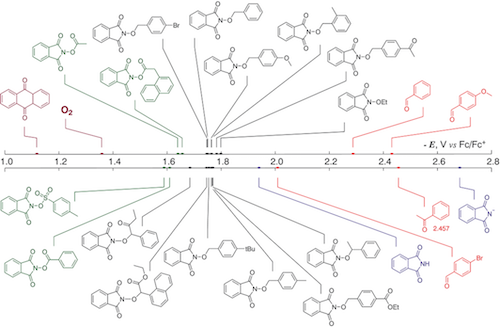
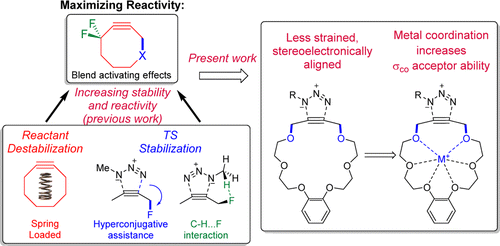
180. Anomeric Effect, Electrostatics, and Hyperconjugation: Lessons from a Classic Stereoelectronic Phenomenon. I. V. Alabugin, L. Kuhn, N. V. Krivoshchapov, P. Mehaffy, M.G. Medvedev. Chem. Soc. Rev., 2021, 50, 10212-10252.
179. Stereoelectronic Power of Oxygen in Control of Chemical Reactivity: the Anomeric Effect is not Alone. I. V. Alabugin, L. Kuhn, M. G. Medvedev, N. V. Krivoshchapov, V. A. Vil’, I. A. Yaremenko, P. Mehaffy, M. Yarie, A. O. Terent’ev, M.A. Zolfigol. Chem. Soc. Rev., 2021, 50, 10253-10345.
178. Marriage of Peroxides and Nitrogen Heterocycles: Selective Three-Component Assembly, Peroxide-Preserving Rearrangement, and Stereoelectronic Source of Unusual Stability of Bridged Azaozonides. I. A. Yaremenko, Y. Y. Belyakova, P. S. Radulov, R. A. Novikov, M. G. Medvedev, N. V. Krivoshchapov, A. A. Korlyukov, I. V. Alabugin, A. O. Terent’ev. J. Am. Chem. Soc., 2021, 143, 6634-6648.
177.
Mapping C-H···M interactions in confined spaces: (α-ICyDMe)Au, Ag, Cu complexes reveal
176. Stalling chromophore synthesis of the fluorescent protein Venus reveals the molecular basis of the final oxidation step. Venus. H. S. Auhima, B. L. Grigorenko, T. Harris, I. V. Polyakov, G.d.P. Gomes, I. V. Alabugin, P. J. Rizkallahg, A. V. Nemukhin, D. D. Jones. Chem. Science, 2021,12, 7735-7745.
175. How to Review a Paper. I. V. Alabugin (invited article), ACS Chemical Health & Safety, 2021, 28, 1, 14-18
174. Organocatalytic sulfoxidation. S. C. Davidson, G. d. P. Gomes, L. R. Kuhn, I. V. Alabugin, A. R. Kennedya, N. C. O. Tomkinson. Tetrahedron, 2021
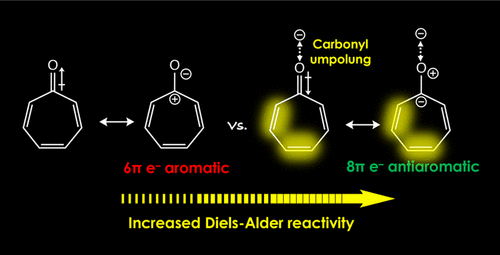
2020 173. Antiaromaticity Gain Activates Tropone and Nonbenzenoid Aromatics as Normal-Electron-Demand Diels–Alder Dienes. Karas, L. J.; Campbell, A. T.; Alabugin, I. V.; Wu, J. I. Org. Lett., 2020, 22, 7083 - 7087.
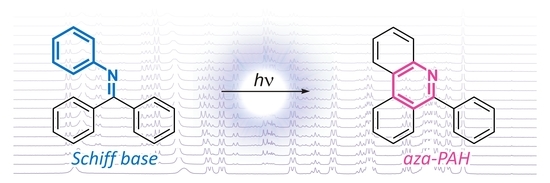
172. Oxidative Photocyclization of Aromatic Schiff Bases in Synthesis of Phenanthridines and Other Aza-PAHs. Kos, M.; Žádný, J.; Storch, J.; Církva, V.; Cuřínová, P.; Sýkora, J.; Císařová, I.; Kuriakose, F.; Alabugin, I. V. Int. J. Mol. Sci., 2020, 21, 5868.
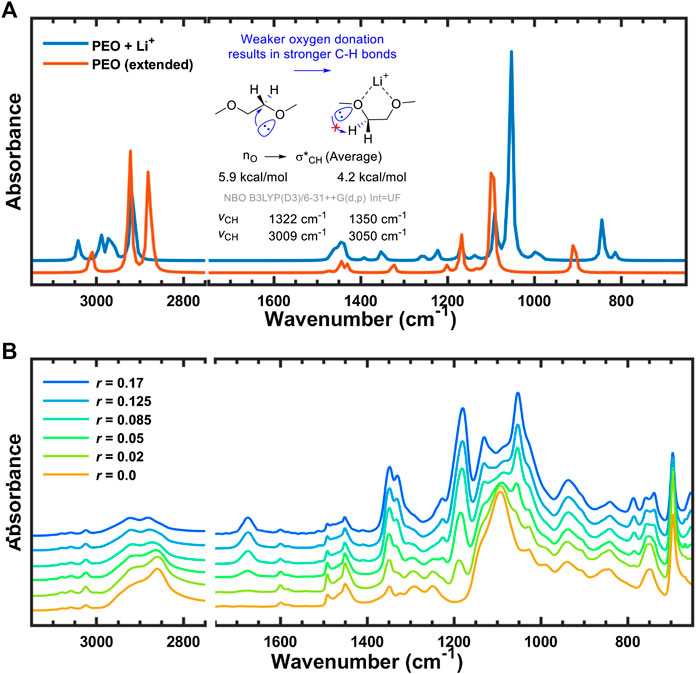
171. Lithium Salt Dissociation in Diblock Copolymer Electrolyte Using Fourier Transform Infrared Spectroscopy. K. Kim, L. Kuhn, I. V. Alabugin, D. T. Hallinan Jr., Frontiers in Energy Research, 2020.
170. How to Build Rigid Oxygen-Rich Tricyclic Heterocycles from Triketones and Hydrogen Peroxide: Control of Dynamic Covalent Chemistry with Inverse α-Effect. I. A. Yaremenko, P. S. Radulov, M. G. Medvedev, N. V. Krivoshchapov, Yu. Yu. Belyakova, A. A. Korlyukov, A. I. Ilovaisky, A. O. Terent’ev, I. V. Alabugin, J. Am. Chem. Soc, 2020, 142, 14588 - 14607.
169. Determination of the pKa values of trans-Resveratrol by Singular Value Decomposition. Comparison with Theory. Zimányi, L.; Thekkan, S.; Eckert, B.; Condren, A. R.; Dmitrenko, O.; Kuhn, L. R.; Alabugin, I. V.; Saltiel, J. J. Phys. Chem. A, 2020, 124, 6294 - 6302.
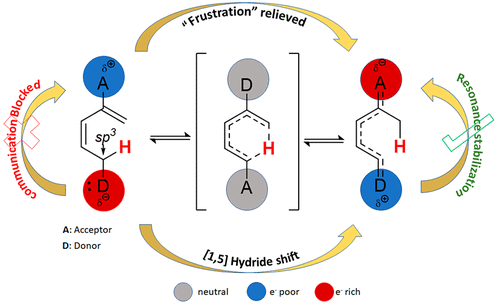
168. [1,5]-Sigmatropic Shifts Regulated by Built-in Frustration. Vidhani, D.; Gillett, J.; Cusido, J.; Alabugin, I.V. J. Phys. Chem. A, 2020, 124, 6016 - 6028.
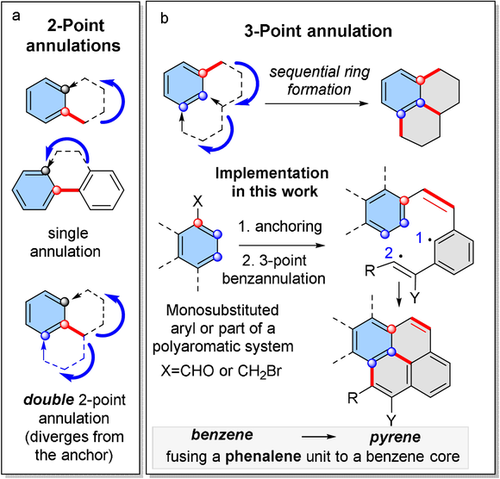
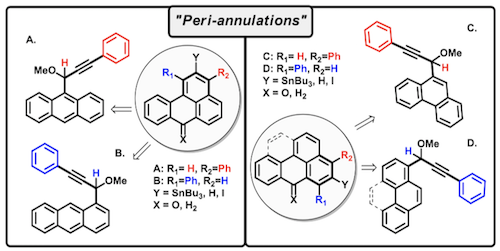
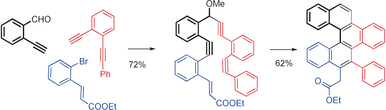
167. Phenalenannulations: three-point double annulation reactions that convert benzenes into pyrenes. R. K. Kawade, C. Hu, N. R. Dos Santos, N. Watson, X. Lin, K. Hanson, I. V. Alabugin. Angew. Chem. Int. Ed., 2020, 59, 14352 - 14357.
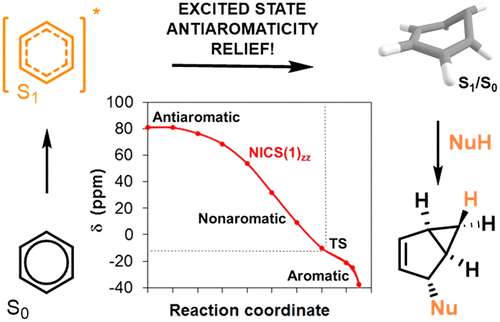
166. On the Impact of Excited State Antiaromaticity Relief in a Fundamental Benzene Photoreaction Leading to Substituted Bicyclo[3.1.0]hexenes. T. Slanina, R. Ayub, J. Toldo, J. Sundell, W. Rabten, M. Nicaso, I. V. Alabugin, I. Fernández Galván, A. Kumar Gupta, R. Lindh, A. Orthaber, R. Lewis, G. Grönberg, J. Bergman, H. Ottosson, J. Am. Chem. Soc, 2020, 142, 10942-10954.
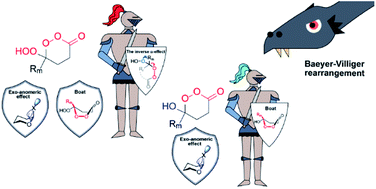
165. Synthesis of unstrained Criegee intermediates: inverse a-effect and other protective stereoelectronic forces can stop Baeyer-Villiger rearrangement of ɣ-hydroperoxy-ɣ-peroxylactones. V. A. Vil’, Y. A. Barsegyan, L. Kuhn, M. V. Ekimova, E. A. Semenov, A. A. Korlyukov, A. O. Terent’ev, I. V. Alabugin, Chem. Science, 2020, 11, 5313 - 5322.
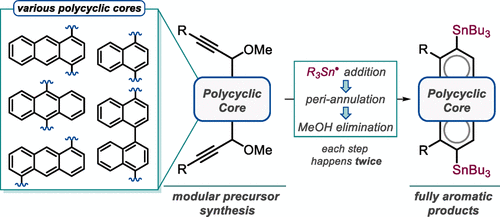
164. Twofold π-Extension of Polyarenes via Double and Triple Radical Alkyne peri-Annulations: Radical Cascades Converging on the Same Aromatic Core. E. Gonzalez-Rodriguez, M. A. Abdo, G. dos Passos Gomes, S. Ayad, F. D. White, N. P. Tsvetkov, K. Hanson, I. V. Alabugin, J. Am. Chem. Soc, 2020, 142, 8352-8366.
163. Testing the Limits of Radical-Anionic CH-Amination: a 10-Million-Fold Decrease in Basicity Opens a New Path to Hydroxyisoindolines via a Mixed C-N/C-O-Forming Cascade. Q. Elliott, G. Gomes, C. J. Evoniuk, I. V. Alabugin, Chem. Science, 2020, 11, 6539 - 6555.
162. Negative charge as a lens for concentrating antiaromaticity in twisted polyaromatics: taking advantage of a pentagonal “defect” and helicene strain for reductive annulation. Z. Zhou, R. K. Kawade, Z. Wei, F. Kuriakose, R. Gershoni-Poranne, M. A. Petrukhina, I. V. Alabugin, Angew. Chem. Int. Ed, 2020, 59, 1256-1262.
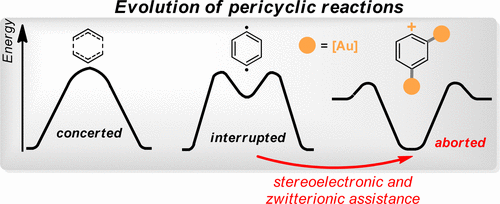
2019 161. Controlled Evolution of the Cope Rearrangement: Transition from Concerted to Interrupted and Aborted Pericyclic Reactions Regulated by a Switch Built from an Intramolecular Frustrated Lewis Pair. D. V. Vidhani, I. V. Alabugin, J. Org. Chem., 2019, 84, 14844-14853.
160. Strain and stereoelectronics in cycloalkyne click chemistry. T. Harris, I. V. Alabugin, Mendeleev Communications, 2019, 29, 237-248.
159. Peroxycarbenium ions as the “gatekeepers” in reaction design: assistance from inverse alpha-effect in three-component ß-alkoxy-ß-peroxylactones synthesis. V. A. Vil’, Y. A. Barsegyan, D. V. Barsukov, A. A. Korlyukov, I. V. Alabugin, A. O. Terent’ev, Chemistry A European Journal, 2019, 63, 14460-14468.
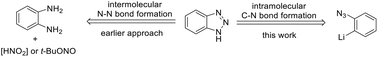
158. Making Endo-Cyclizations Favorable Again: Conceptually New Synthetic Approach to Benzotriazoles via Azide Group Directed Lithiation/Cyclization of 2-Azidoaryl Bromides. A. A. Ageshina, G. A. Chesnokov, M. A. Topchiy, I. V. Alabugin, M. S. Nechaev, A. F. Asachenko, Org. Biomol. Chem., 2019, 17, 4523-4534.
157. CO2 or SO2: Should It Stay, or Should It Go? Gomes, G.; Wimmer, A.; Smith, J.; König, B.; Alabugin, I.V., J. Org. Chem., 2019, 84, 6232-6243.
156. Alkynes as synthetic equivalents of ketones and aldehydes: a hidden entry into carbonyl chemistry. I. V. Alabugin, E. Gonzalez-Rodriguez, R. K. Kawade, A. Stepanov, S. F. Vasilevsky, Molecules, 2019, 24, 1036-1073.
155. Stereoelectronic Influence of a “Spectator” Propargylic Substituent Can Override Aromaticity Effects in Radical Peri-cyclizations on Route to Expanded Polyaromatics. A. Hughes; G. Gomes; I. V. Alabugin, J. Org. Chem., 2019, 84, 1853-1862.
154. Upconversion of Reductants. M. A. Syroeshkin, F. Kuriakose, E. A. Saverina, V. A. Timofeeva, M. P. Egorov, I. V. Alabugin, Angew. Chem. Int. Ed., 2019, 57, 5532-5550.
153. Optimizing amine-mediated alkyne-allene isomerization to improve benzannulation cascades: synergy between theory and experiments. G. Gomes, A. E. Morrison, G. B. Dudley, I. V. Alabugin, Eur. J. Org. Chem., 2019, 512-518.
152. Hyperconjugation. I. V. Alabugin, G. Gomes, M. A. Abdo, WIREs: Comput. Mol. Sci., 2019, 9, e1389.
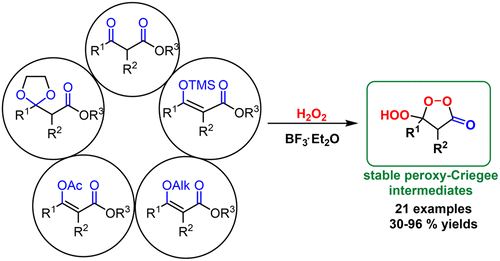
2018 151. Five roads that converge at the cyclic peroxy-Criegee intermediates: BF3-catalyzed synthesis of ß-hydroperoxy- ß-peroxylactones. Vil’, V. A.; Gomes, G. P.; Ekimova, M. V.; Lyssenko, K. A.; Syroeshkin, M. A.; Nikishin, G. I.; Alabugin, I. V.; Terent’ev, A. O. J. Org. Chem., 2018, 83, 13427-13445.
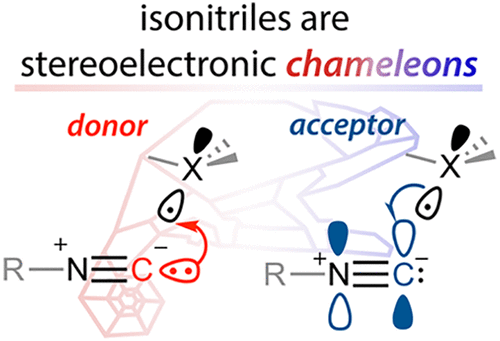
150. Isonitriles as Stereoelectronic Chameleons: The Donor-Acceptor Dichotomy in Radical Additions. G. Gomes, Y. Loginova, S. Z. Vatsadze, I V. Alabugin. J. Am. Chem. Soc., 2018, 140, 14272-14288.
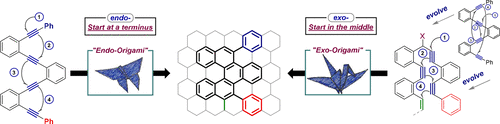
149. Alkyne Origami: Folding Oligoalkynes into Polyaromatics. I. V. Alabugin, E. Gonzalez-Rodriguez.Acc. Chem. Res., 2018, 51, 1206-1219.
148. Ozone-Free Synthesis of Ozonides: Assembling Bicyclic Structures from 1,5-Diketones and Hydrogen Peroxide. Yaremenko, I.; Gomes, G.; Radulov, P.; Belyakova, Y.; Vilikotskiy, A.; Vil', V.; Korlyukov, A.; Nikishin, G.; Alabugin, I. V.; Terent'ev, A. O. J. Org. Chem., 2018, 83, 4402-4426.
147. Radical Alkyne Peri-annulations for Synthesis of Functionalized Phenalenes, Benzanthrenes, and Olympicene. N. P. Tsvetkov, E. Gonzalez-Rodriguez, A. Hughes, G. dos Passos Gomes, F. D. White, F. Kuriakose, I. V. Alabugin Angew. Chem. Int. Ed., 2018, 57, 3651-3655.
146. Interrupted Baeyer-Villiger Rearrangement: Building A Stereoelectronic Trap for the Criegee Intermediate. V. A. Vil', G. dos Passos Gomes, O. V. Bityukov, K. A. Lyssenko, G. I. Nikishin, I. V. Alabugin, A. O. Terent'ev Angew. Chem. Int. Ed., 2018, 57, 3372-3376.
145. Photochemical activation of enediyne warheads: A potential tool for targeted antitumor therapy. P. Bhattacharya, A. Basak, A. T. Campbell, I. V. Alabugin Molecular Pharmaceutics, 2018, 15, 768-797.
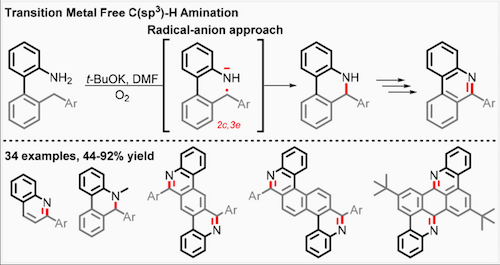
2017 144. Coupling C-H activation, N-H deprotonation and oxidation: metal-free C(sp3)-H aminations with unprotected anilines. Evoniuk, C. J.; Gomes, G. P.; Hill, S.; Satoshi, F.; Hanson, K.; Alabugin, I. V. J. Am. Chem. Soc., 2017, 139, 16210–16221
143. Substituent effects on stereoselectivity of dihalocarbene reactions with cyclohexadiene and on the reactivity of bis-dihalocyclopropanes in electrophilic nitrations on route to pyrimidine N-oxides. Sedenkova, K. N.; Averina, E. B.; Grishin, Y. K.; Kolodyazhnaya, J. V.; Rybakov, V. B.; Kuznetsova, T. S.; Hughes, A.; Gomes, G. P.; Alabugin, I. V.; Zefirov, N. S., Org. Biom. Chem., 2017, 15, 9433–9441
142. Twisted chiral cyclodecynes and remote activation of click reactivity. Harris, T.; Gomes, G. P.; Ayad, S.; Clark, R.; Lobodin, V. V.; Tuscan, M.; Hanson, K.; Alabugin, I. V. Chem, 2017, 3 (4), 629–640
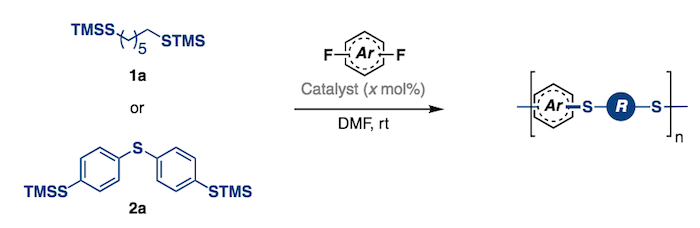
141. Organocatalyzed Synthesis of Fluorinated Poly(aryl thioethers). Park, N. H.; Gomes, G. P.; Fevre, M.; Jones, G. O.; Alabugin, I. V.; Hedrick, J. L. Nature Communications, 2017, 8, Article Number: 166
140. Electrochemical behavior of N-oxyphthalimides: Cascades initiating self-sustaining catalytic reductive N-O bond cleavage.. Syroeshkin, M. A.; Krylov, I. B.; Hughes, A. M.; Alabugin, I. V.; Nasybullina, D. V.; Sharipov, M. Yu.; Gultyai, V. P.; Terent'ev, A. O., J. Phys. Org. Chem., 2017, e3744
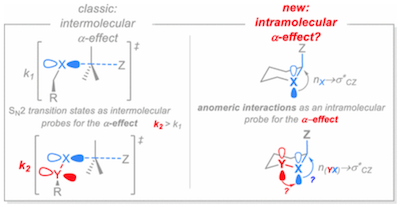
139. Stereoelectronic Interactions as a Probe for the Existence of the Intramolecular alpha-Effect. Juaristi, E.; Gomes, G. P.; Terent'ev, A. O.; Notario, R.; Alabugin, I. V. J. Am. Chem. Soc., 2017, 139 , 10799–10813
138. Formaldehyde-Extruding Homolytic Aromatic Substitution via C->O Transposition: Evolution of a Contaminating Side-Reaction to Traceless-Linker access to Congested Biaryl Bonds. Poonptana, P.; Gomes, G. P.; Hurrle, T.; Chardon, K.; Brase, S.; Masters, K-S.; Alabugin, I. V., Chem. Eur. J., 2017, 23, 9091–9097.
137. Cyclooctyne. Harris, T.; Alabugin, I. V. e–EROS Encyclopedia of Reagents for Organic Synthesis, 2017, 1–3, DOI: 10.1002/047084289X.rn02079
136. Photoredox-Initiated Radical Cascades Enabling Collective Synthesis of 33 Natural Products.. Alabugin, I. V. & Harris, T. Chem, 2017, 2, 753–755.
135.
Stereoelectronic Control in the Ozone–Free Synthesis of Ozonides.
Gomes, G. P.; Yaremenko, I. A.; Radulov, P. S.; Novikov, R. A.; Chernyshev, V. V.; Korlyukov, A. A.; Nikishin, G. I.; Alabugin, I. V.; Terent'ev, A. O.,
Angew. Chem. Int. Ed., 2017, 56, 4955–4959.
134.
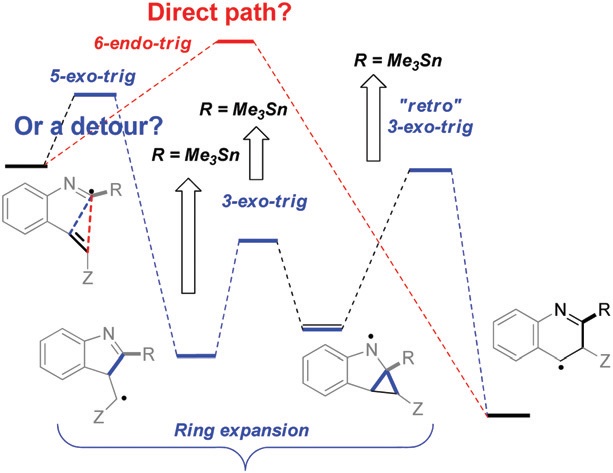
Changing the path of least resistance, or access to endo-dig products via a sequence of three exo-trig transition states: electronic effects in homoallyic ring expansion cascades of alkenyl isonitriles.
Gomes, G. P.; Evoniuk, C.; Ly, M.; Alabugin, I. V.,
Org. Biomol. Chem., 2017, 15, 4135–4143.
133.
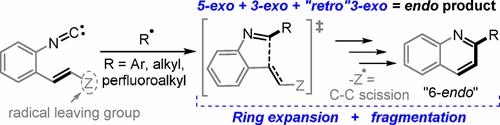
Coupling radical homoallylic expansions with C–C fragmentations for the synthesis of heteroaromatics: Quinolines from reactions of o–alkenylarylisonitriles with aryl, alkyl and perfluoroalkyl radicals.
Evoniuk, C.; Gomes, G. P.; Ly, M.; White, F.; Alabugin, I. V.,
J. Org. Chem., 2017, 82, 4265–4278.
132.
Drawing Catalytic Power from Charge Separation: Stereoelectronic and Zwitterionic Assistance in the Au(I)-Catalyzed Bergman Cyclization.
Gomes, G. P.; Alabugin, I. V.,
J. Am. Chem. Soc., 2017, 139, 3406–3416.
131.
Full Cleavage of C≡C Bond in Electron-Deficient Acetylenes Via Reaction with Ethylenediamine.
Vasilevsky, S. F.; Davydova, M. P.; Mamatyuk, V. I.; Tsvetkov, N.; Hughes, A.; Baranov, D. S.; Alabugin, I. V.,
Aust. J. Chem., 2017, 70, 421–429.
130.
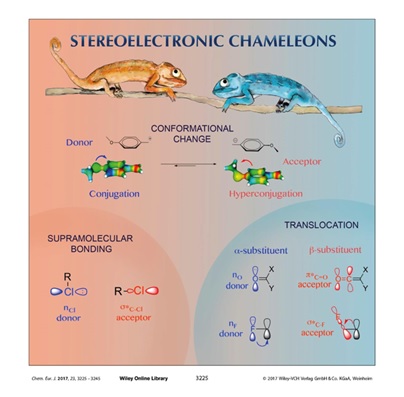
Stereoelectronic Chameleons: The Donor-Acceptor Dichotomy of Functional Groups.
Vatsadze, S. Z.; Loginova, Y. D.; Gomes, G.; Alabugin, I. V.,
Chem. Eur. J., 2017, 23, 3225–3245. Frontispiece: http://onlinelibrary.wiley.com/doi/10.1002/chem.201781461/full
129.
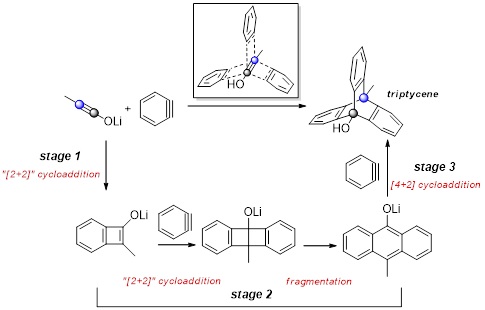
Regioselective One–pot Synthesis of Triptycenes via Triple-Cycloadditions of Arynes to Ynolates.
Umedu, S.; Gomes, G. P.; Sakae, M.; Yoshinaga, T.; Matsumoto, K.; Iwata, T.; Alabugin, I. V.; Shindo, M.,
Angew. Chem. Int. Ed., 2017, 56, 1298–1302. (Selected by Synfacts as "Synfact of the month")
2016
128.
Orbital Crossings Activated Through Electron Injection: Opening Communication between Orthogonal Orbitals in Anionic C1–C5 Cyclizations of Enediynes.
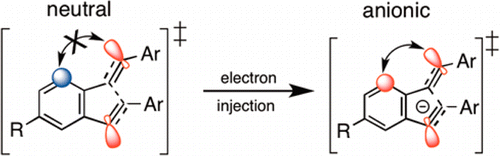
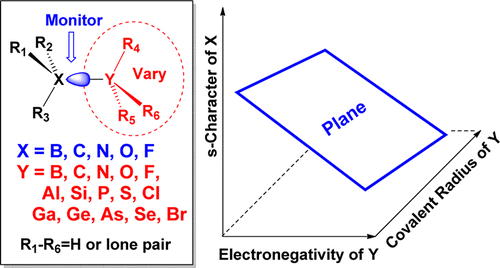
Peterson, P.; Shevchenko, N.; Breiner, B.; Manoharan, M.; Lufti, F.; Delaune, J.; Kingsley, M.; Kovnir, K.; Alabugin, I. V.,
J. Am. Chem. Soc., 2016, 138, 15617–15628.
127.
Alkynes as Linchpins for the Additive Annulation of Biphenyls: Convergent Construction of Functionalized Fused Helicenes.
Mohamed, R. K.; Mondal, S.; Guerrera, J. V.; Eaton, T.M.; Albrecht-Schmitt, T. E.; Shatruk, M.; Alabugin, I. V.,
Angew. Chem. Int. Ed., 2016, 55, 12054–12058.
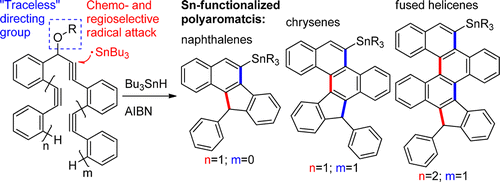
126.
Combining Traceless Directing Groups with Hybridization Control of Radical Reactivity: from Skipped Enynes to Defect-Free Hexagonal Frameworks.
Pati, K.; Gomes, G.; Alabugin, I. V.,
Angew. Chem. Int. Ed., 2016, 55, 11633–11636.
125.
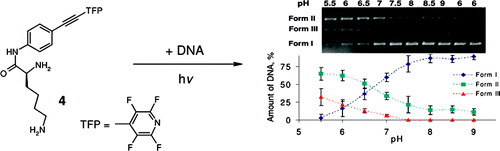
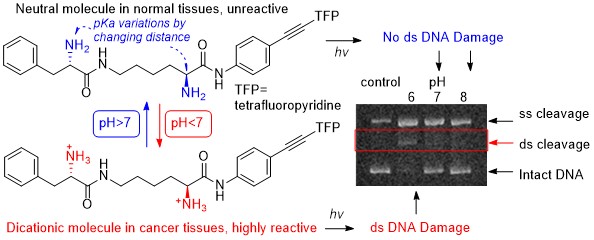
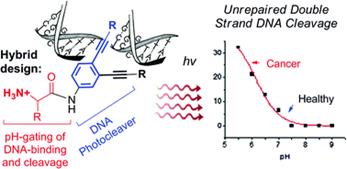
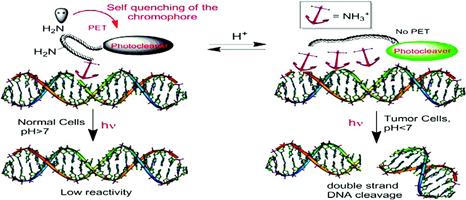
Optimizing Protonation States for Selective Double Strand DNA Photocleavage in Hypoxic Tumors: pH-Gated Transitions of Lysine Dipeptides.
Kaya, K.; Roy, S.; Nogues, J. C.; Echeverri, J. C. R.; Sokolikj, Z.; Zorio, D. A. R.; Alabugin, I. V.,
J. Med. Chem., 2016, 59, 8634–8647.
124.
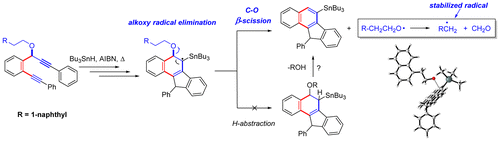
Domino Fragmentations in Traceless Directing Groups of Radical Cascades: Evidence for the Formation of Alkoxy Radicals via C–O Scission.
Harris, T.; Gomes, G.; Clark, R. J.; Alabugin, I. V.,
J. Org. Chem., 2016, 81, 6007–6017.
123.
Double C-H Amination by Consecutive SET Oxidations.
Evoniuk, C. J.; Hill, S. P.; Hanson, K.; Alabugin, I. V.,
Chem. Commun., 2016, 52, 7138–7141.
122.
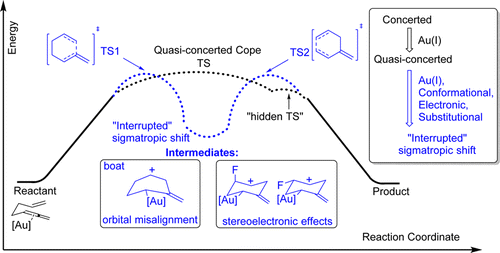
Gold(I)‐catalyzed allenyl Cope rearrangement: evolution from asynchronicity to trappable intermediates assisted by stereoelectronic switching.
Vidhani, D.; Krafft, M.; Alabugin, I. V.,
J. Am. Chem. Soc., 2016, 138, 2769–2779.
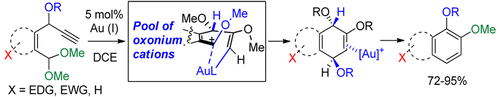
121.
Fused Catechol Ethers from Gold (I)–Catalyzed Intramolecular Reaction of Propargyl Ethers with Acetals.
Pati, K.; Gomes, G.; Harris, T.; Alabugin, I. V.,
Org. Lett., 2016, 18, 928–931.
120.
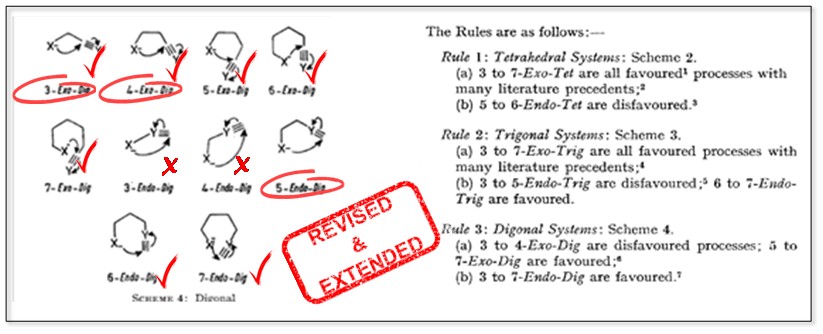
The Baldwin Rules: Revised and Extended.
Gilmore, K; Mohamed, R. K.; Alabugin, I. V.,
WIREs Comput. Mol. Sci., 2016, 6, 487–514.
2015
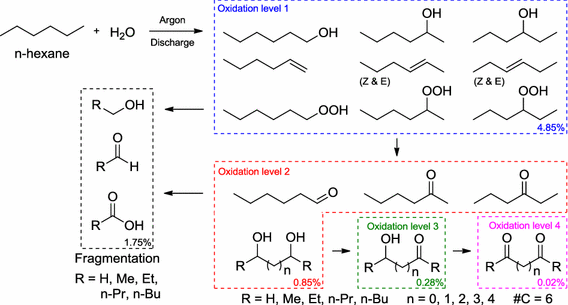
119.
Oxidized derivatives of n-hexane from a water/argon continuous flow electrical discharge plasma reactor.
Bresch, S.; Wandell, R.; Wang, H.; Alabugin, I. V.; Locke, B.,
Plasma Chemistry and Plasma Processing, 2015, 36 (2), 553–584.
118.
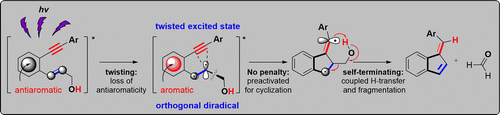
The Missing C1-C5 Cycloaromatization Reaction: Triplet State Antiaromaticity Relief and Self-terminating Photorelease of Formaldehyde for Synthesis of Fulvenes from Enynes.
Mohamed, R. K.; Mondal, S.; Jorner, K.; Faria Delgado, T.; Ottosson, H.; Alabugin, I. V.,
J. Am. Chem. Soc., 2015, 137 (49), 15441–15450. 117.
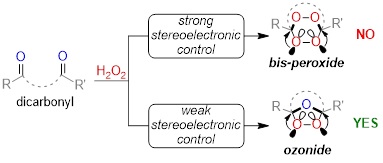
Stereoelectronic source of the anomalous stability of bis-peroxides.
Gomes, G. P.; Vil', V.; Terent'ev, A.; Alabugin, I. V.,
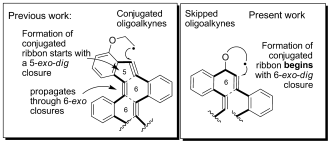
Chem. Sci., 2015, 6, 6783–6791. 116.
Reaction of α,β-alkynylketones with β-amino alcohols: pseudoephedrine- assisted cleavage of triple bond via formal internal redox process.
Vasilevsky, S. F.; Davydova, M. P.; Mamatuyk, V. I.; Pleshkova, N. V.; Fadeev, D. S.; Alabugin, I. V.,
Mendeleev Communications, 2015, 25, 377–379. 115.
Synthesis of Functionalized Phenanthrenes via Selective Oxidative Radical Cyclization.
Pati, K.; Michas, C.; Allenger, D.; Piskun, I.; Coutros, P.S.; Gomes, G.P.; Alabugin, I. V.,
J. Org. Chem., 2015, 80(23), 11706–11717. 114.
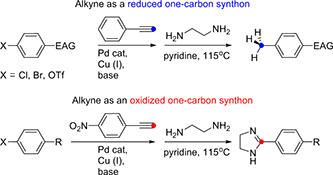
Coupling cyclizations with fragmentations for the preparation of heteroaromatics: a new route to quinolines from o-alkenyl arylisocyanides.
Evoniuk, C. J.; Ly, M.; Alabugin, I. V.,
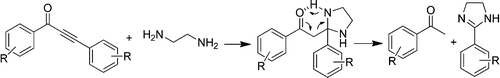
Chem. Commun., 2015, 51, 12831–12834. 113.
Alkenes as Alkyne Equivalents in Radical Cascades Terminated by Fragmentations: Overcoming Stereoelectronic Restrictions on Ring Expansions For the Preparation of Expanded Polyaromatics.
Mohamed, R.; Mondal, S.; Gold, B.; Evoniuk, C. J.; Banerjee, T.; Hanson, K.; Alabugin, I. V.,
J. Am. Chem. Soc., 2015, 137, 6335–6349. 112.
UV and Sunlight Driven Photoligation of Quantum Dots: Understanding the Photochemical Transformation of the Ligands.
Aldeek, F.; Hawkins, D.; Palomo, V.; Safi, M.; Palui, G.; Dawson, P. E.; Alabugin, I. V.; Mattoussi, H.,
J. Am. Chem. Soc., 2015, 137, 2704–2714. 111.
Conformational Flexibility of Fused Tetracenedione Propellers Obtained from One-Pot Reductive Dimerization of Acetylenic Quinones.
Vasilevsky, S. F.; Baranov, D. S.; Mamatyuk, V. I.; Fadeev, D. S.; Gatilov, Y. V.; Stepanov, A. A.; Vasilieva, N. V.; Alabugin, I. V.,
J. Org. Chem., 2015, 80, 1618–1631. 110.
Traceless Directing Groups in Radical Cascades: From Oligoalkynes to Fused Helicenes without Tethered Initiators.
Pati, K.; Gomes, G. P.; Harris, T.; Hughes, A.; Phan, H.; Banerjee, T.; Hanson, K.; Alabugin, I. V.,
J. Am. Chem. Soc., 2015, 137, 1165–1180. 109.
Opening Enediyne Scissors Wider: pH–Dependent DNA Photocleavage by meta–Diyne Lysine Conjugates.
Kaya, K.; Johnson, M.; Alabugin, I. V.,
Photochemistry and Photobiology (the Michael Kasha issue), 2015, 91, 748–758. 108.
Orbital Hybridization: a Key Electronic Factor in Control of Structure and Reactivity.
Alabugin, I. V.; Bresch S.; Gomes, G. P.,
J. Phys. Org. Chem., 2015, 28, 147–162. (invited review for the ISRIUM-2014 issue) 2014 107.
Click chemistry on diterpenes: anti–inflammatory activity of the acetylenic derivatives of levopimaric acid and products of their transformations.
Vasilevsky, S. F.; Baranov, D. S.; Govdi, A. I.; Sorokina, I. V.; Tolstikova, T. G.; Tolstikov, G. A.; Alabugin, I.V.,
ARKIVOC, 2014, 2014, 145–157. 106.
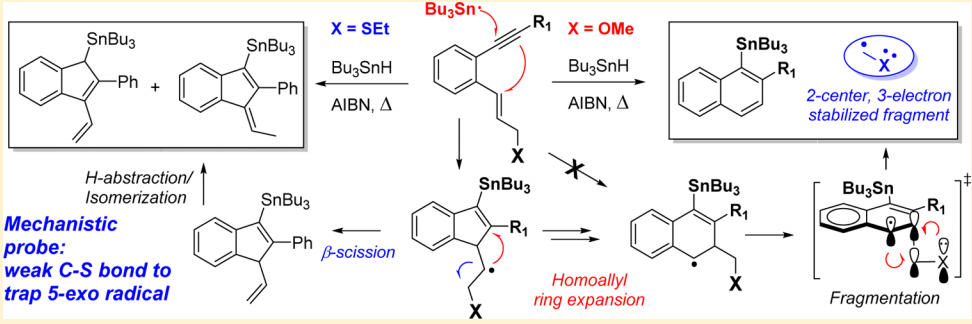
Rerouting Radical Cascades: Intercepting the Homoallyl Ring Expansion in Enyne Cyclizations via C–S Scission.
Mondal, S.; Gold, B.; Mohamed, R.; Phan, H.; Alabugin, I. V.,
J. Org. Chem., 2014, 79, 7491–7501. 105.
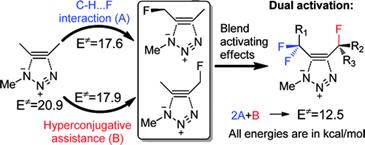
Alkynyl Crown Ethers as a Scaffold for Hyperconjugative Assistance in Non-catalyzed Azide-Alkyne Click Reactions: Ion Sensing through Enhanced Transition State Stabilization.
Gold, B.; Batsomboon, P.; Dudley, G. B.; Alabugin, I. V.,
J. Org. Chem., 2014, 79, 6221–6232. 104.
Hybridization Trends for Main Group Elements and Expanding the Bent's Rule Beyond Carbon: More than Electronegativity.
Alabugin, I. V.; Bresch S.; Manoharan, M.,
J. Phys. Chem., 2014, 118, 3663–677. 103.
Synthesis of Substituted Biaryls Through Gold–Catalyzed Petasis–Ferrier Rearrangement of Propargyl Ethers.
Pati, K.; Alabugin, I. V.,
Eur. J. Org. Chem., 2014, 19, 3986–3990. 102.
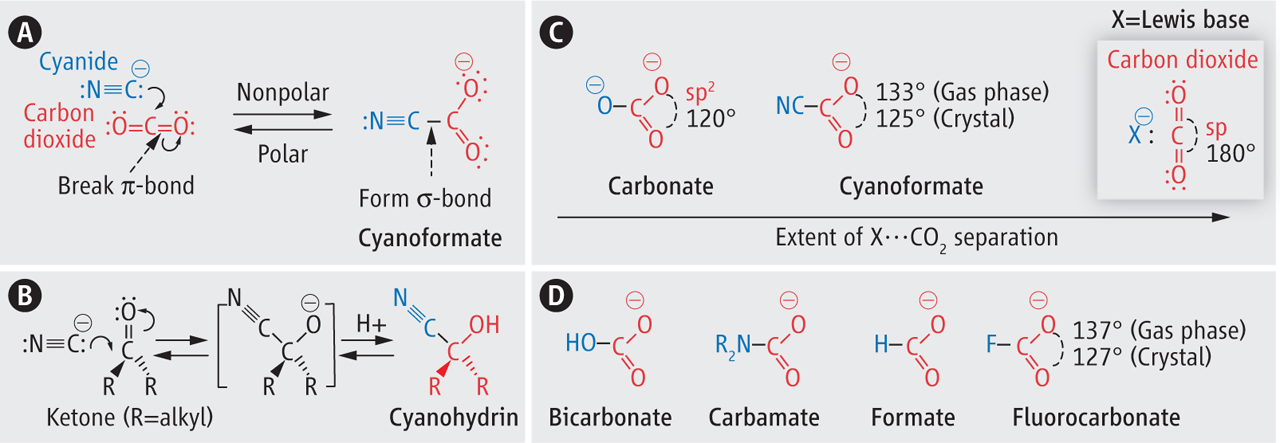
A CO2 Cloak for the Cyanide Dagger.
Alabugin, I. V.; Mohamed, R.,
Science, 2014, 344, 45–46. 101.
Design of Leaving Groups in Radical C–C Fragmentations: Through–Bond 2c‐3e Interactions in Self–Terminating Radical Cascades.
Mondal, S.; Gold, B.; Mohamed, R. K.; Alabugin, I. V.,
Chem. Eur. J., 2014, 20, 8664–8669. 100.
Formation of Alcohols and Carbonyl Compounds from Hexane and Water in a Liquid Film Plasma Reactor.
Wandell, R. J.; Bresch S.; Hsieh K.; Alabugin, I. V.; Locke B. R.,
IEEE Transactions on Plasma Science, 2014, 42, 1195–1205. 99.
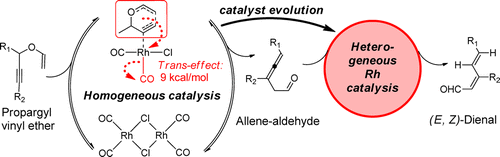
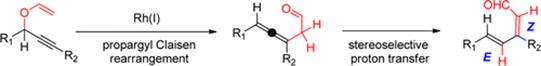
Mechanistic Insights into the Rh(I)–Catalyzed Propargyl Claisen/Prototropic Rearrangement Cascade: Stereoelectronic Role of Trans–Effect on the Pericyclic Process and a Shift from Homogeneous to Heterogeneous Catalysis During a One–Pot Reaction.
Vidhani, D. V.; Krafft, M. E.; Alabugin, I. V.,
J. Org. Chem., 2014, 79, 352–364. 98.
Exo‐Dig Radical Cascades of Skipped Enediynes: Building a Naphthalene Moiety within a Polycyclic Framework.
Pati, K.; Hughes, A. M.; Phan, H.; Alabugin, I. V.,
Chem. Eur. J., 2014, 20, 390–393. 2013 97.
Finding the right path: Baldwin "Rules for Ring Closure" and stereoelectronic control of cyclizations.
(Invited "Viewpoint").
Alabugin, I. V.; Gilmore, K.
Chem. Commun., 2013, 49, 11246 – 11250. 96.
Drawing from a pool of radicals for the design of selective enyne cyclizations.
Mondal, S.; Mohamed, R. K.; Manoharan, M.; Phan, H.; Alabugin, I. V.
Org. Lett., 2013, 15, 5650–5653. 95.
Concerted Reactions that Produce Diradicals and Zwitterions: Electronic, Steric, Conformational and Kinetic Control of Cycloaromatization Processes.
Mohamed, R. K.; Peterson, P. W.; Alabugin, I. V.
Chem. Rev., 2013, 7089–7129. (Special Issue on Reactive Intermediates,
Cover). 94.
Stereocontrolled synthesis of (E, Z)–dienals via tandem Rh(I)–catalyzed rearrangement of propargyl vinyl ethers.
Vidhani, D. V.; Krafft, M. E.; Alabugin, I. V.,
Org. Lett., 2013, 15, 4462–4465. 93.
"Two functional groups in one package": designing cascade transformations of alkynes. (Invited "Synopsis")
Alabugin, I. V.; Gold, B.
J. Org. Chem., 2013, 78, 7777–7784. 92.
Stereoelectronic Umpolung: Converting a p-Donor into a σ-Acceptor via Electron Injection and a Conformational Change.
Peterson, P. W.; Shevchenko, N.; Alabugin, I. V.
Org. Lett. 2013, 15, 2238. 91.
How to Lose a Bond in Two Ways: The Diradical/Zwitterion Dichotomy in Cycloaromatization Reactions. Invited "Microreview"
Peterson, P. W.; Mohamed, R. K.; Alabugin, I. V.
Eur. J. Org. Chem., 2013, 2013, 2505–2527. 90.
Combining ligand design with photo–ligation to provide compact, colloidally stable and easy to conjugate quantum dots.
Zhan, N.; Palui, G.; Grise, H.; Tang, H.; Alabugin, I.; Mattoussi, H.
ACS Applied Materials & Interfaces (invited). 2013, 5, 2861–2869. 89.
Moderating strain without sacrificing reactivity: Design of fast and tunable non–catalyzed alkyne-azide cycloadditions via stereoelectronically controlled transition state stabilization.
Gold, B.; Dudley, G. B.; Alabugin, I. V.
J. Amer. Chem. Soc., 2013, 135, 1558–1569. 88.
Gold (I)–Catalyzed Propargyl Claisen Rearrangement: Catalytic Pathways from Higher Energy Au(I)–Substrate Complexes and Reactant Deactivation via Unproductive Complexation.
Vidhani, D. V.; Cran, John W.; Krafft M. E.; Alabugin, I. V.
Org. Biomol. Chem., 2013, 11, 1624 – 1630.
(Journal Cover) 87.
Convenient, Ambient Temperature Generation of Sulfonyl Radicals for Addition to Alkynes.
Gilmore, K.; Gold, B.; Clark, R.; Alabugin, I.V.
Aust. J. Chem., 2013, 66, 336–340 (Invited paper for a special issue).
86.
Gold(I)–Catalyzed Claisen Rearrangement of Allenyl Vinyl Ethers: Missing Transition States Revealed through Evolution of Aromaticity, Au(I) as an Oxophilic Lewis Acid, and Lower Energy Barriers from a High Energy Complex.
Vidhani, D. V.; Cran, John W.; Krafft M. E.; Manoharan, M.; Alabugin, I. V.
J. Org. Chem. 2013, 78, 2059–2073 (H. E. Zimmerman Issue).
85.
Divergent Cyclizations of 1–R–Ethynyl–9,10–anthraquinones: Use of Thiourea as a "S2–-" Equivalent in an "Anchor–Relay" Addition Mediated by Formal C–H Activation.
Baranov, D.; Gold, B.; Vasilevsky, S.; Alabugin, I. V.
J. Org. Chem. 2013, 78, 2074–2082 (H. E. Zimmerman Issue).
84.
Reinvestigation of "Single–Crystal X–ray Structure of 1,3–dimethylcyclobutadiene".
Shatruk, M., Alabugin, I. V.
Chemistry – Eur. Journal, 2013, 19, 4942–4945.
2012 83.
Alabugin, I. V.; Gilmore, K. (2012)
Unusual Radical Cyclizations.
Encyclopedia of Radicals in Chemistry, Biology and Materials, C. Chatgilialoglu; A. Studer (Eds.). John Wiley & Sons Ltd, Chichester, UK, pp 693–728. 82.
Alabugin, I. V.; Yang, W.–Y.; Pal. R. (2012)
Enediyne photochemistry.
CRC Handbook of Organic Photochemistry and Photobiology, Griesbeck, A.; Oelgemöller, M.; Ghetti, F. (Edss); Taylor & Francis, Boca Raton, FL. 81.
Polyaromatic Ribbon– Benzofuran Fusion via Consecutive Endo Cyclizations of Enediynes.
Byers, P. M.; Rashid, J. I.; Mohamed, R. K.; Alabugin, I. V.
Org. Lett., 2012, 14, 6032–6035. 80.
Photo–Induced Phase Transfer of Luminescent Quantum Dots to Aqueous Media.
Palui, G.; Avellini, T.; Zhan, N.; Pan, F.; Gray, D.; Alabugin, I. V.; Mattoussi, H.
J. Amer. Chem. Soc. 2012, 134, 16370–16378.
79.
Strain Control in Nucleophilic Cyclizations: Reversal of exo–Selectivity in Cyclizations of Hydrazides of Acetylenyl Carboxylic Acids by Annealing to a Pyrazole Scaffold.
Vasilevsky, S. F.; Gold, B.; Mikhailovskaya, T. F.; Alabugin, I. V.
J. Phys. Org. Chem. (invited article), 2012, 25, 998–1005.
78.
Aromatic Transition States in Non-Pericyclic Reactions: Anionic 5–Endo Cyclizations are as Aborted Sigmatropic Shifts.
Gilmore, K.; Manoharan, M.; Wu, J.; Schleyer, P. v. R; Alabugin, I. V.
J. Amer. Chem. Soc. 2012, 134, 10584–10594.
77.
Tandem Nucleophilic Addition/Fragmentation of Vinylogous Acyl Nonaflates for the Synthesis of Functionalized Alkynes, with a Key Mechanistic Insight.
Batsomboon, P.; Gold, B.; Alabugin, I. V.; Dudley, G. B.
Synthesis 2012, 44, 1818‐1824 (invited article).
76.
Polyaromatic Ribbons from Oligo–Alkynes via Selective Radical Cascade: Stitching Aromatic Rings with Polyacetylene Bridges.
Byers, P.; Alabugin, I. V.
J. Am. Chem. Soc. 2012, 134, 9609–9614. (Highlighted in SYNFACTS)
75.
Hybrids of Amino Acids and Acetylenic DNA–Photocleavers: Optimizing Efficiency and Selectivity for Cancer Phototherapy.
Breiner, B.; Kaya, K.; Roy, S.; Yang, W.–Y.; Alabugin, I. V.
Org. Biomol. Chem. 2012, 10, 3974–3987. (Invited "Perspective").
74.
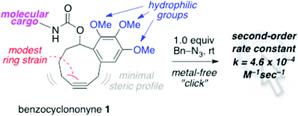
Strain-Promoted Azide–Alkyne Cycloadditions of Benzocyclononynes.
Tummatorn, J.; Batsomboon, P.; Clark, R. J.; Alabugin, I. V.; Dudley, G. B.
J. Org. Chem. 2012, 77, 2093–2097.
73.
Selective Transition State Stabilization via Hyperconjugative and Conjugative Assistance: Stereoelectronic Concept for Copper–Free Click Chemistry.
Gold, B.; Schevchenko, N.; Bonus, N.; Dudley, G. B.; Alabugin, I. V.
J. Org. Chem. 2012, 77, 75–89.
2011 72.
Yang, W.–Y.; Roy, S.; A. R.; Phrathep, B.; Rengert, Z.; Alabugin, I. V.
Engineering Multiple pH–Gated Transitions for Selective and Efficient Double Strand DNA Photocleavage in Hypoxic Tumors.
J. Med. Chem., 2011, 54, 8501–8516.
71.
Baranov, D.S.; Vasilevsky, S. F.; Gold, B.; Alabugin, I. V.
Urea as a Solvent and Reagent for the Addition/Cyclization/Fragmentation Cascades Leading to 2–R–7H-dibenzo[de,h]quinolin–7– one Analogues of Aporphinoid Alkaloids.
RSC Adv., 2011, 1, 1745–1750.
70.
Stepanov, A.A.; Gornostaev, L. M.; Vasilevsky, S. F.; Arnold, E. V.; Mamatyuk, V.I.; Fadeev, D.S.; Gold, B.; Alabugin, I. V.
Chameleonic reactivity of vicinal diazonium salt of acetylenyl–9,10–antraquinones: Synthetic application towards two heterocyclic targets.
J. Org. Chem. 2011, 76, 8737–8748.
69.
Roy, S.; Davydova, M. P.; Pal, R.; Gilmore, K.; Tolstikov, G. A.; Vasilevsky, S. F.; Alabugin, I. V.
Dissecting Alkynes: Full Cleavage of Polarized C≡C Moiety via Sequential Bis–Michael Addition/Retro–Aldol Cascade.
J. Org. Chem., 2011, 76, 7482–7490.
68.
Gilmore, K.; Alabugin, I. V.
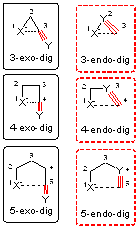
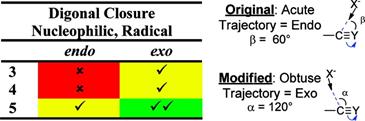
Cyclizations of Alkynes: Revisiting Baldwin's Rules for Ring Closure.
Chem. Rev. >2011. 111, 6513–6556.
67.
Alabugin, I. Gilmore, K.; Manoharan, M.
Rules for Anionic and Radical Ring Closure of Alkynes.
J. Am. Chem. Soc. 2011, 133, 12608–12623.
66.
Yang, W.-Y.; Marrone, S. A.; Minors, N.; Zorio, D. A. R.; Alabugin, I. V.
Fine–tuning alkyne cycloadditions: Insights into photochemistry responsible for the double-strand DNA-cleavage via structural perturbations in diaryl alkyne conjugates.
Beilst. J. Org. Chem., 2011, 7, 813–823. (Invited article in special issue on "Photocycloadditions and Photorearrangements").
65.
Baroudi, A.; Alicea, J.; Flack, P.; Kirincich, J.; Alabugin, I. V.
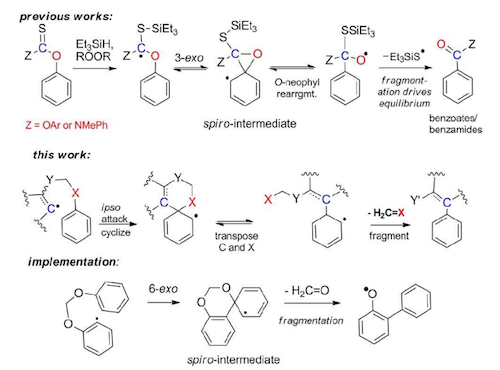
Radical O→C Transposition: a Metal-Free Process for Conversion of Phenols into Benzoates and Benzamides,
J. Org. Chem. 2011, 76, 1521–37. (JOC "Featured Article". Highlighted in http://www.organic-chemistry.org/Highlights/2011/17October.shtm).
64.
Alabugin, I. V.; Gilmore, K.; Peterson, P.
Hyperconjugation.
WIREs Comput. Mol. Sci., 2011, 1, 109-141 (Invited Review).
63.
Vasilevsky, S.F.; Govdi, A. I.; Sorokina, I. V.; Tolstikova, T. G.; Baev, D. S.; Tolstikov, G. A.; Mamatuyk, V. I.; Alabugin, I. V.
Rapid Access to New Bioconjugates of Betulonic Acid via Click Chemistry.
Bioorganic and Medicinal Chemistry Lett. 2011, 21, 62-65.
2010 62.
Alabugin, I. V.; Gold, B.; Shatruk, M.; Kovnir, K.
"
On the "Single-Crystal X-ray Structure of 1,3-dimethylcyclobutadieneby Confinement in a Crystalline Matrix""
Science, 2010, 330, 1047.
For comments and discussion, see C&E News,
Nature Chemistry.
RSC Publishing
as well as blogs by H. Rzepa and
S. Bachrach. 61.
Pal, R.; Clark, R.J.; Manoharan, M.; Alabugin I. V.
Fast Oxy-Cope Rearrangements of Bis-Alkynes: Competition with Central C-C bond Fragmentation and Incorporation in Tunable Cascades Diverging from a Common bis-Allenic Intermediate.
J. Org. Chem., 2010, 75, 8689-8692.
60.
Baroudi, A.; Flack, P.; Alabugin, I. V.
Metal-Free Transformation of Phenols into Substituted Benzamides: A Highly Selective Radical 1,2-O,C transposition in O-Aryl-N-phenyl-thiocarbamates.
Chemistry Eur. Journal, 2010, 16, 12316-12320.
59.
Yang, W.-Y.; Cao, Q.; Callahan, C.; Galvis, C.; Sang, A. Q.-X.; Alabugin, I. V.
Intracellular DNA Damage by Lysine-Acetylene Conjugates.
Journal of Nucleic Acids, 2010, Article ID 931394. DOI:10.4061/2010/931394 (Special issue on DNA damage and repair).
58.
Baroudi, A.; Alicea, J.; Alabugin, I. V.
Radical 1,2-O,C Transposition for Conversion of Phenols into Benzoates via O-Neophyl Rearrangement/Fragmentation Cascade.
Chemistry Eur. Journal, 2010, 16, 7683-7687.
57.
Baroudi, A.; Mauldin, J.; Alabugin, I. V.
Conformationally Gated Fragmentations and Rearrangements Promoted by Interception of the Bergman Cyclization through Intramolecular H-Abstraction: A Possible Mechanism of Auto-Resistance to Natural Enediyne Antibiotics?
J. Am. Chem. Soc., 2010, 133, 967-979.
56.
Velizhanin, K. A.; Zeidan, T. A.; Alabugin, I. V.; Smirnov, S.
Single Molecule Conductance of Bipyridyl Ethynes: The Role of Surface Binding Modes.
J. Phys. Chem. A, 2010, 114, 14189-14193.
2005-2009 55.
Vasilevsky, S.F.; Govdi, A. I.; Shults, E. E.; Shakirov, M. M.; Tolstikov, G. A.; Alabugin, I. V.
Efficient Synthesis of the Betulonic Acid-Acetylene Hybrids and Their Hepatoprotective and Anti-Inflammatory Activity.
Bioorganic & Medicinal Chemistry, 2009, 17, 5164-5169.
http://dx.doi.org/10.1016/j.bmc.2009.05.059.
54.
Vasilevsky, S. F.; Mikhailovskaya, T. F.; Mamatyuk, V. I.; Bogdanchikov, G. A.; Manoharan, M.; Alabugin, I. V.
Tuning Selectivity of Anionic Cyclizations: Competition between 5-Exo- and 6-Endo-dig Closures of Hydrazides of o-Acetylenyl Benzoic Acids and Based-catalyzed Fragmentation/Recyclization of the Initial 5-Exo-Dig Products.
J. Org. Chem., 2009, 74, 8106-8117.
http://pubs.acs.org/doi/abs/10.1021/jo901551g
53.
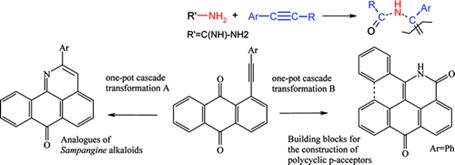
Vasilevsky, S. F.; Baranov, D. S.; Mamatyuk, V.I.; Gatilov, Y. V.; Alabugin, I. V. An Unexpected Rearrangement which Disassembles Alkyne Moiety Through Formal Nitrogen Atom Insertion between Two Acetylenic Carbons and Related Cascade Transformations: New Approach to Sampagine Derivatives and Polycyclic Aromatic Amides,
J. Org. Chem., 2009, 74, 6143-6150.
http://pubs.acs.org/doi/abs/10.1021/jo9008904.
52.
Breiner, B.; Kovalenko, S. V.; Ben, C.; Singh, M.; LeGrand, S. N.; Sang, A. Q.-X.; Strouse, G. F., Copland, J. A.; Alabugin I. V.
C-Lysine Conjugates: pH-Controlled Light-Activated Reagents for Efficient Double Stranded DNA Cleavage with Implications for Cancer Therapy,
J. Am. Chem. Soc., 2009, 131, 11458-11470.
http://pubs.acs.org/doi/abs/10.1021/ja902140m.
51.
Vasilevsky, S. F.; Govdi, A. I.; Shult'ts, E. E., Shakirov, M. M.; Alabugin I. V., Tolstikov, G. A.
Synthesis of the First Acetylene Derivatives of Betulonic Acid.
Proc. Russ. Acad. Sci. (Doklady Chemistry), 2009, 424, 631-635.
http://www.springerlink.com/content/j78h637p866u3305. 50.
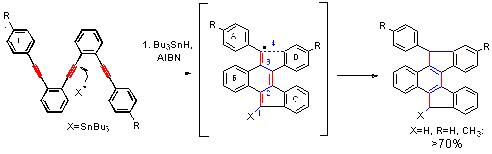
Alabugin, I. V.;& Gilmore, K.; Patil, S.; Manoharan, M.; Kovalenko, S. V.; Clark, R. J.; Ghiviriga, I.
Radical Cascade Transformations of Tris-(o-aryleneethynylenes) into Substituted Benzo[a]indeno[2,1-c]fluorenes,
J. Am. Chem. Soc., 2008, 130, 11535-11545.
http://pubs.acs.org/doi/abs/10.1021/ja8038213.
49.
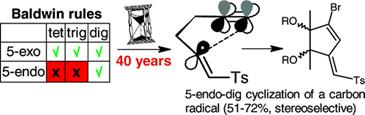
Alabugin, I. V.; Timokhin, V. I.; Abrams, J. N.; Manoharan, M.; Ghiviriga, I., Abrams, R.
In Search of Efficient 5-Endo-dig Cyclization of a Carbon-Centered Radical: 40 Years from a Prediction to Another Success for the Baldwin Rules.
J. Am. Chem. Soc., 2008, 130, 10984-10995.
http://pubs.acs.org/doi/abs/10.1021/ja801478n.
48. Vasilevsky, S. F.; Krivenko, O. L.;
Gorelik, V. R.; Alabugin, I. V.
Synthetic And Mechanistic Aspects Of Cross-Coupling Of Nitroxyl Radicals Of 3-Imidazoline Series With Terminal Alkynes,
Tetrahedron, 2008, 64, 8807-8814.
http://www.sciencedirect.com/science/article/pii/S0040402008012210.
47.
Nenajdenko, V. G.; Shevchenko, N. E.; Balenkova, E. S.; Alabugin I.V. (2007).
Organochalcogen Multication Species in F. A. Devillanova (Ed.),
Handbook of Chalcogen Chemistry, 417-453, RSC Publishing, Great Britain. 46.
Alabugin, I. V.; Breiner, B.; Manoharan, M. (2007).
Electronic Effects in Cycloaromatization Reactions: The Melting Pot of Theory and Experiment in J. Richard (Ed.),
Advances in Physical Organic Chemistry, 42, 1-35. Elsevier,
http://dx.doi.org/10.1016/S0065-3160(07)42001-9. 45.
Breiner, B.; Schlatterer, J. C.; Kovalenko, S. V.; Greenbaum, N. L.; Alabugin I. V.
"DNA Damage-Site Recognition by Lysine Conjugates",
Proc. Natl. Acad. Sci., 2007, 104, 13016-13021.
Press release.
44.
Vasilevsky, S. F.; Krivenko, O. L.; I. V. Alabugin
"Oxidative coupling of alkynes mediated by nitroxyl radicals under Sonogashira conditions and Pd-free catalytic approach to stable radicals of 3-imidazoline family with triple bonds"
Tetrahedron Lett., 2007, 48, 8246-8249.
43.
Alabugin I. V.; Manoharan, M.; Buck, M.; Clark, R. J.
"Substituted Anilines: The Tug-Of-War between Pyramidalization and Resonance Inside and Outside of Crystal Cavities",
THEOCHEM, 2007, 813, 21-27.
42.
Vasilevsky S.F.; Gornostaev L.M.; Stepanov A.A.; Arnold E.V.; Alabugin I. V.
"Unmasking of aminoanthroquinone moiety through a ring-opening in the presence of copper salts and a subsequent cross-coupling/recyclization cascade",
Tetrahedron Lett., 2007, 48, 1867-1870.
41.
Alabugin I. V.; Manoharan, M. "Rehybridization as a General Mechanism for Maximizing Chemical and Supramolecular Bonding and a Driving Force for Chemical Reactions",
J. Comp. Chem. (Invited paper for the special issue dedicated to the 90th anniversary of the Chemical Bond), 2007, 28, 373-390.
http://onlinelibrary.wiley.com/doi/10.1002/jcc.20524/pdf.
40.
Breiner, B.; Schlatterer, J. C.; Kovalenko, S. V.; Greenbaum, N. L.; Alabugin I. V.
"Protected 32P-Labels in Deoxyribonucleotides: Investigation of Sequence Selectivity of DNA Photocleavage by Enediyne-, Fulvene-, and Acetylene-Lysine Conjugates."
Angew. Chem. Int. Ed., 2006, 45, 3666-3670.
http://onlinelibrary.wiley.com/doi/10.1002/anie.200504479/pdf.
39.
Pickard IV, F. C.; Shepherd, R. L.; Gillis, A. E.; Dunn, M. E.; Feldgus, S.; Kirschner, K. N.; Shields, G C.; Manoharan, M.; Alabugin I. V.
"Ortho Effect in the Bergman Cyclization: Electronic and Steric Effects in Hydrogen Abstraction by 1-Substituted Naphthalene 5,8-Diradicals",
J. Phys. Chem. A, 2006, 110, 2517-2526.
38.
Zeidan, T.; Kovalenko, S. V.; Manoharan, M.; Alabugin I. V.
"Ortho Effect in the Bergman Cyclization: Comparison of Experimental Approaches and Dissection of Cycloaromatization Kinetics",
J. Org. Chem., 2006, 71, 962-975.
37.
Zeidan, T.; Manoharan, M.; Alabugin I. V.
"Ortho Effect in the Bergman Cyclization: Interception of p-Benzyne Intermediate by Intramolecular Hydrogen Abstraction",
J. Org. Chem., 2006, 71, 954-961.
36.
Kauffman, J. F.; Turner, J. M.; Alabugin, I. V.; Breiner, B.; Kovalenko, S. V.; Badaeva, E. A.; Masunov A., Tretiak, S.
"Two Photon Excitation of Substituted Enediynes"
J. Phys. Chem. A 2006, 110, 241-251.
35.
Alabugin I.V.; Manoharan, M.
"Thermodynamic and Strain Effects in the Competition Between 5-Exo-dig and 6-Endo-Dig Cyclizations of Vinyl and Aryl Radicals"
2005, 2005, 127, 12583-12594.
34.
Zeidan, T.; Clark, R. J.; Kovalenko, S. V.; Ghiviriga, I.; Alabugin I. V.
"Triplet acetylenes as Synthetic Equivalents of 1,2-Dicarbenes. II. New Supramolecular Scaffolds from Photochemical Cycloaddition of Diarylacetylenes to 1,4-Cyclohexadienes",
Chemistry Eur. Journal, 2005, 11, 4953-4962.
http://onlinelibrary.wiley.com/doi/10.1002/chem.200500180/abstract.
33.
Alabugin I.V.; Manoharan, M.;
"5-Endo-dig Cyclizations – the "Poor Cousins" of the Radical Cyclization Family"
J. Am. Chem. Soc. 2005, 127, 9534-9545.
32.
Kovalenko, S. V.; Alabugin I. V.
"Lysine-Enediyne Conjugates as Photochemically Triggered DNA Double-Strand Cleavage Agents".
Chem. Comm. 2005, 1444-1446.
31.
Zeidan, T.; Kovalenko, S. V.; Manoharan, M.; Clark, R. J.; Ghiviriga, I.; Alabugin I. V.
"Triplet acetylenes as Synthetic Equivalents of 1,2-Dicarbenes. Phantom n,p* State Controls Reactivity in Triplet Photocycloaddition",
J. Am. Chem. Soc. 2005, 127, 4270-4285.
30.
Peabody, S.; Breiner, B.; Kovalenko, S. V.; Patil, S.; Alabugin I. V.
"Synthesis of Selectively Deuterated Fulvenes and Indenes from Enediynes"
Org. Biomol. Chem. 2005, 3, 218-221.
2000-2004 29.
Alabugin I. V.; Manoharan, M.
"Effect of Double Hyperconjugation on the Apparent Donor Ability of s-Bonds: Insights From the Relative Stability of d-Substituted Cyclohexyl Cations",
J. Org. Chem. 2004, 69, 9011-9024. 28.
Alabugin I. V.; Manoharan, M.; Weinhold F.
"Blue-Shifted and Red-Shifted Hydrogen Bonds in Hypervalent Rare-Gas FRg-HY Sandwiches"
J. Phys. Chem. A. 2004, 108, 4720. 27.
Kovalenko, S. V.; Peabody, S.; Manoharan, M.; Clark, R. J., Alabugin I.V.
"5-Exo-dig Radical Cyclization of Enediynes: The First Synthesis of Tin-Substituted Benzofulvenes"
Org. Lett. 2004, 6, 2457-2460. 26.
Alabugin I.V.; Manoharan, M.; Zeidan, T. A.
"Homoanomeric Effects in Saturated Heterocycles"
J. Am. Chem. Soc. 2003, 125, 14014-14031. 25.
Alabugin I.V.; Manoharan, M.; Breiner, B.; Lewis, F.
"Control of Kinetics and Thermodynamics of [1,5]-Shifts by Aromaticity: A View Through the Prism of Marcus Theory"
J. Am. Chem. Soc. 2003, 125, 9329-9342. *24.
Alabugin I.V.; Manoharan, M.; Peabody S.; Weinhold, F.
"The Electronic Basis of Improper Hydrogen Bonding: A Subtle Balance of Hyperconjugation and Rehybridization."
J. Am. Chem. Soc. 2003, 125, 5973-5987
(one of 20
most cited 2003 JACS papers) 23.
Alabugin I. V.; Manoharan, M.
"Radical-Anionic C1-C5 and C1-C6 Cyclizations of Enediynes: Remarkable Substituent Effects in Cyclorearomatization Reactions."
J. Am. Chem. Soc. 2003, 125, 4495-4509.
22.
Nenajdenko, V. G.; Shevchenko, N. E.; Balenkova, E. S.; Alabugin I.V.
"1,2-Dications in Organic Main Group Systems",
Chem. Rev. 2003, 103, 229-282. 21.
Alabugin I. V.; Manoharan, M.
"Reactant Destabilization in the Bergman cyclization and Rational Design of Light and pH-Activated Enediynes"
J. Phys. Chem. A 2003, 107, 3363-3371. 20.
Alabugin I. V.; Kovalenko, S.V.
"C1-C5 Photochemical Cyclization of Enediynes"
J. Am. Chem. Soc. 2002, 124, 9052-9053. 19.
Alabugin I. V.; Manoharan, M.; Kovalenko, S.V.
"Tuning Rate of the Bergman Cyclization of Benzannelated Enediynes with Ortho Substituents",
Org. Letters 2002, 4, 1119-1122. 18.
Alabugin I. V.; Zeidan, T. A.
"Stereoelectronic Effects and General Trends in Hyperconjugative Acceptor Ability of σ Bonds."
J. Am. Chem. Soc. 2002, 124, 3175-3185. 17.
Alabugin I. V.
"Stereoelectronic interactions in cyclohexane, 1,3-dioxane, 1,3-oxathiane and 1,3-dithiane: W-effect, σ C-X ↔ σ * C-H interactions, anomeric effect - what is really important?"
J. Org. Chem. 2000, 65, 3910-3919
(one of 50 most cited 2000-2006 JOC papers) Invited
Articles (not peer reviewed)
Alabugin, I. V. Book Review: "
Hydrogen Bonding in Organic Synthesis"
by Wiley-VCH Weinheim/Germany, Editor: P. M. Pihko, 2009, J. Am. Chem. Soc. 2010, 132, 6863–6866. (invited).
http://pubs.acs.org/doi/abs/10.1021/ja103155b
16. Zimmerman H.E.; Alabugin I. V.
"Energy Distribution and Redistribution and Chemical Reactivity. The Generalized Delta Overlap-Density Method for Ground State and Electron Transfer Reactions. A New Quantitative Counterpart of Electron Pushing"
J. Am. Chem. Soc. 2001, 123, 2265-2270.
15. Zimmerman H.E.; Alabugin I.V.
"Energy Distribution and Redistribution and Chemical Reactivity. Mechanistic and Exploratory Organic Photochemistry."
J. Am. Chem. Soc. 2000, 122, 952-953.
14. Zimmerman H.E.; Alabugin I.V., Smolenskaya, V.N.
"Exploratory and Theoretical Host-Guest Photochemistry. Control of Reactivity with Host Variation and Theoretical Treatment with Stress Shaped Reaction Cavity."
Tetrahedron 2000, 56, 6821-6831.
13. Zimmerman H.E.; Alabugin I.V., Chen W., Zhu, Z.
"Dramatic Effects of Crystal Morphology on Solid State Reaction Course. Control by Crystal Disorder. Mechanistic and Exploratory Organic Photochemistry."
J. Am. Chem. Soc. 1999, 121, 11930-11931.
12. Alabugin I.V.; Brel V.K.
"Phosphorus-Containing Allenes - Structure and Reactions with Electrophilic Reagents (Review)."
Russ. Chem. Rev. 1997, 66, 225-245.
11. Nenajdenko V.G.; Vertelezkij P.V.; Koldobskij A.B.; Alabugin I.V.; Balenkova E.S.
"Oxidative Properties of Triflic Anhydride - Oxidation of Alcohols and Sulfides."
J. Org. Chem. 1997, 62, 2483-2486.
10. Alabugin I.V.; Brel V.K.
"Alkenylsulfenylchlorides. II. Interaction of g,g-Disubstituted Phosphorus-Containing Allenes with Sulfur dichloride."
Phosphorus, Sulfur and Silicon 1996, 119, 61-75.
9. Alabugin I.V.; Sereda G.A.; Abramkin E.V.; Brel V.K.; Zyk N.V.; Zefirov N.S.
"Synthesis of Heterocyclic and Acyclic Derivatives of 1,2-Alkadienylphosphonic Acids Using Potassium Dichloroiodate(I)."
J. Org. Chem (Russ.) 1996, 32, 1400-1403.
8. Alabugin I.V.; Brel V.K.; Zyk N.V.; Zefirov N.S.
"SO3-Mediated Reaction of Phenylselenenylamide with 1,2-Alkadienylphosphonates ."
Russian Chemical Bulletin 1996, 45, 739-740.
7. Zyk N.V.; Beloglazkina E.K.; Alabugin I.V.; Kutateladze A.G.; Zefirov N.S.
"S-Tosylthiosufenylamides as Electrophilic Sulfosulfenylating Reagents."
Moscow State University Bulletin (Ser.2, Chemistry) 1996, 31, 68-71.
6. Alabugin I.V.; Brel V.K.
"Interaction of 3-Methyl-1,2-Butadienylphosphonic Acid Dichloride with SCl2."
Journal of General Chemistry (Russia) 1995, 65, 1670-1672.
5. Alabugin I.V.; Sereda G.A.; Abramkin E.V.; Brel V.K.; Zyk N.V.; Zefirov N.S.
"Interaction of Potassium Dichloroiodate(I) with 1,2-Alkadienylphosphonic Acids Derivatives."
Proceedings of Russian Academy of Science (Doklady Akademii Nauk) 1995, 345, 487-489.
4. Chekhlov A.N.; Alabugin I.V.; Brel V.K.; Zefirov N.S.
"Molecular-Structure of Crystalline Product and Stereochemistry of Addition of 5,5-Dimethyl-2-Methoxy-2-Oxo-4-Chlorothio-1,2-Oxaphosphol-3-Ene to Cyclohexene."
Proceedings of Russian Academy of Science (Doklady Akademii Nauk) 1994,
3. Zyk N.V.; Alabugin I.V.; Kutateladze A.G.; Kice J.L.; Zefirov N.S.
"Electrophilic Sulfamatoselenenation of Olefins."
Proceedings of Russian Academy of Science (Doklady Akademii Nauk) 1994, 337, 208-210.
2. Alabugin I.V.; Brel V.K.; Chekhlov A.N.; Zefirov N.S.; Stang P.J.
"Alkenylsulfenylchlorides - Synthesis and AdE Reactions of 2-Alkoxy-2-Oxo-3-R-4-Chlorothio-1,2-Oxaphosphol-3-Enes."
Tetrahedron Letters 1994, 35, 8275-8278.
1. Alabugin I.V.; Brel V.K.; Zefirov N.S.
"Synthesis of 2-Alkoxy-5,5-Dimethyl-4-Chlorothio-1,2-Oxaphosphol-3-Enes."
Journal of General Chemistry (Russia) 1993, 63, 2387-2389. Updated April 2017 |
|||||

























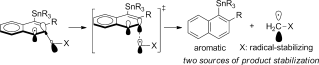


![Graphical abstract: Urea as an organic solvent and reagent for the addition/cyclization/fragmentation cascades leading to 2-R-7H-dibenzo[de,h]quinolin-7-one analogues of Aporphinoid alkaloids](publications_files/image033.jpg)
![[1860-5397-7-93-3]](publications_files/image038.gif)