
Archimedes

Experiment 1 A Submarine Adventure: Density Saves the Day |
Introduction/Background
 Archimedes |
Around 250 B.C., Archimedes, a Greek mathematician, was charged by the King of Syracuse to determine if the content of his crown was pure gold. The king had reason to believe the craftsman of the crown was cheating him by charging for pure gold but using silver. An idea came to him while he was bathing. He noticed that the amount of water that flowed out was equal to the space his body occupied in the bathtub. This observation would lead him to test the contents of the crown. Gold has a density of 19.30 g/ cm3 and silver equals 10.50 g/ cm3. This means that silver takes up more volume than an equal mass of gold. Archimedes tested the density by placing the craftsman's crown and a crown of pure gold in two tubs of water. He discovered that the Craftsman's crown displaced more water per gram than the pure crown. It turned out the craftsman was a sham. It was said that Archimedes ran through the streets of Sicily screaming "Eureka!" ("I have found it!") He had discovered the concept of density. |
Everyone has been submerged in water many times in his or her lives. Archimedes made a second observation; he felt lighter when he was floating. This feeling is now known as buoyancy. Buoyancy is the force that keeps objects afloat in water or liquids. It is related to density. If a substance is less dense than the liquid in which it is placed, it will float. If it is not, it will sink.
| A feather would float on water while a rock would sink. The feather would float because it is less dense than water where the rock is more dense. Steel is certainly more dense than water. One could ask the question how a submarine that weighs several tons would be able to float. A submarine takes advantage of air space found within it. The ship's total density is less than water; therefore, it will remain afloat. The captain of the sub opens the hull to allow water in and causes the sub to submerge into the water. Once the water is released, submarine comes back to the surface. A more practical example of density is oil and water. Oil and water do not mix with each other. The oil will float to the top, which would indicate that it is less dense than water. Another example to consider is orange juice. If orange juice is allowed to go undisturbed, some of the contents will fall out of solution and accumulate at the bottom. The insoluble contents are more dense than the solution. This is the reason for the instructions labeled on the container that tells you to shake before use. |
 dive! dive! |
Key Concepts
Density is used to characterize different substances. It is equal to the mass (g) of an object divided by its volume (mL or cm3): d = m/ v = g/ (mL or cm3). We use milliliters (mL) when the volume is a liquid and cm3 when it is a solid.
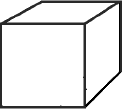
| Example 1: Measuring the density of a solid (see figure 1 below) d = m/v The cube is 2.5 cm on each side. 2.5 cm x 2.5 cm x 2.5 cm or (2.5)3cm3 = 15.625 cm 3 Weigh the solid on an analytical balance and if it weighs 65g then the density is: 65g/15.625 cm3 = 4.16g/cm3 Answer: With proper significant figures our final answer is 4.2g/cm3 |
| Example 2: Measuring the density of a liquid (see figure 2 below) The graduated cylinder below weighs 45.6g. If we add 4.5 mL of water and reweigh it, we will find that it now weighs 50.1g. What is the density of water? The volume of the water is 4.5mL and the mass is simply the mass of the cylinder and the water together minus the cylinder alone. 50.1g - 45.6g = 4.5g d = m/v = 4.5g/4.5mL = 1.0g/mL = 1.0g/cm3 Answer: 1.0g/cm3 |

Glossary
Density = An object's mass divided by its volume. (d = m/V)
Volume = The three dimensional space an object occupies. Often expressed in units of cm3 or milliliters.
Bouyancy = The property of floating on the surface of a liquid, or in a fluid, as in the atmosphere; specific lightness, which is inversely as the weight compared with that of an equal volume of water.
Related Materials
Visionlearning - Density (http://www.visionlearning.com/library/module_viewer.php?c3=1&mid=37&l=)