CHEMISTRY HOME
BLACKBOARD LOGIN
LAB MANUAL HOME
SYLLABUS
| Using the information in the preceding paragraph, design a submarine that will be capable of hovering in the water above the bottom but that will not rise to the surface and give your position away.
- Collect a balloon and 16 metal pellets from the front counter. Make observations regarding the shape, size and color of the balloon and pellets in your lab notebook.
- Take a watch glass from your desk along with the balloon and pellets into the balance room.
- Place the watch glass on the balance and hit the “tare” button to zero the balance with the watch glass on it.
- Place the balloon in the watch glass and record the mass to the 0.001 g in your lab notebook.
- Remove the balloon, re-zero the balance if necessary and then gently place the 16 pellets in the watch glass. Note: you should wipe the pellets off with a kimwipe as you go so that you don’t weigh fingerprints. Record the mass of the pellets to the 0.001 g in your lab notebook.
- Set the balloon and pellets aside for later use and get your 10 mL graduated cylinder out of your locker.
- You need to weigh the graduated cylinder. Take it to the balance room and tare the balance and then place the graduated cylinder on the balance gently. Record the mass to the 0.001 g in your lab notebook.
- Collect ~3 – 5 mL of “ocean” water from the cooler at the front of the room in your weighed graduated cylinder.
- Record the exact volume of water you have collected to the 0.01 mL in your lab notebook.
- Re-weigh the graduated cylinder with the water in it and record the weight to the 0.001 g.
- Calculate the density of the ocean water.
- Using the equation for a sphere, set the equation up to calculate a volume that will have the balloon with the pellets equal in density to the density of the “ocean” water. Solve for r (r = the radius of the balloon in cm).
Ex. “ocean” water density = 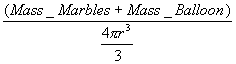
- Put all 16 pellets in your balloon.
- Blow the balloon up so that the diameter of the balloon is twice your calculated r value from step 12.
- Tie off the balloon and take it up to the “ocean” and try it out. A successful submarine doesn’t float on top or sink to the bottom but rather hovers in between. Good Luck!
|
|

