
stone henge

Experiment 4 Half-Lives and Radioactive Waste Disposal |
Introduction/Background
The term half-life is the time required for half of a substance to undergo a process. This can refer to the time required for half of the atoms in a radioactive substance to decay. It can also refer to the time required for half the amount of a drug in a living system (you) to be eliminated by natural processes. Since your lab is centered on radioactive decay, that's what we'll focus on.
The rates of nuclear decay are usually discussed in terms of the half-lives. Each isotope (an isotope is atoms with identical atomic numbers but which differ by number of neutrons) has its own unique half-life. For example, the half-life of strontium-90 is 28.8 years. If you start with 10 grams of 90Sr and wait 28.8 years, you'll have 5 grams left of 90Sr. If another 28.8 years go by, 2.5 grams will remain. Another 28.8 years, and 1.25 grams will be left...and so on. An important concept to remember is that the half-life for any given isotope is always the same. They are unaffected by external conditions such as temperature or pressure. This means that radioactive atoms cannot be made harmless by any chemical reaction or treatment. We just have to allow the nuclei to undergo decay at their characteristic rates.
| Note: The mass that undergoes nuclear decay becomes another element. |
Since the half-life of an atom is constant, it can be used as a clock to determine the ages of different substances. For example carbon-14 has been used for a long time to determine the age of organic materials. In using carbon dating, we assume that the ratio of carbon-14 to carbon-12 in the atmosphere has been constant for the last 50,000 years. 14C is incorporated into CO2, which is then incorporated into plants. When we eat the plants, the 14C is incorporated into our system. Our 14C:12C ratio remains the same as the atmosphere until we die. At this point the ratio decreases. By measuring this decrease and comparing it to the atmosphere ratio, we can estimate the age of something that was once living.
Key Concepts

| Let's do some calculations related to these concepts. Taking our strontium-90 example at the start of the introduction, let's figure out what the half-life would be if we started with 100 grams of strontium-90 and plotted its decay on a graph. Remember the definition of half-life is the time required for 1/2 of a substance to decay. It takes 28.8 years for half of the strontium to decay to 50 grams. This is called one half-life. After 28.8 more years, strontium-90 has decayed to 25 grams. This is called two half-lives. And so on.... An important equation relates the decay constant, k, and half-life, t1/2. | 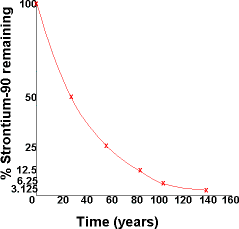 |
| Example 1: Using the equation: Amount remaining = (initial amount) x (1/2)n, calculate the amount of 15N that would be left after 5 minutes of decay, knowing that the half-life of 15N is 1 minute, and you started with 10 grams. Given: Initial amount is 10 grams. Now we just need to calculate how many half-lives (n). Since, one half-life of decay is 1 minute, and we want to know how much is remaining after 5 minutes, then n = 5/1 or 5. Therefore: Amount 15N remaining = (10 grams) x (1/2)5 = 0.3125 grams of 15N |
| Example 2: Calculate the amount of 13C remaining after 3 minutes of decay, if you start with 17 grams, and the half-life of 13C is 20 seconds. Answer: click to reveal |
Glossary
Radioactive = of, caused by, or exhibiting radioactivity .
Isotope = any of two or more species of atoms of a chemical element with the same atomic number and nearly identical chemical behavior but with differing atomic mass or mass number and different physical properties.
Half-Life = the time required for half of something to undergo a process: as a:the time required for half of the atoms of a radioactive substance to become disintegrated.
Nucleus = the positively charged central portion of an atom that comprises nearly all of the atomic mass and that consists of protons and neutrons except in hydrogen which consists of one proton only.
Radii = the plural of RADIUS, which is a line segment extending from the center of a circle or sphere to the circumference or bounding surface.
Related Materials
Half-Life Calculator (http://www.1728.com/halflife.htm)
Radiocarbon WEB-Info (http://www.c14dating.com/index.html)
Radioactivity in Nature (http://www.physics.isu.edu/radinf/natural.htm)