Acid rain is more acidic than normal rain and forms through a complex process of chemical reactions involving air pollution. The two most important pollutants that contribute to the formation of acid rain are oxides of nitrogen and sulfur dioxide, which react with moisture in the atmosphere to form nitric and sulfuric acid.
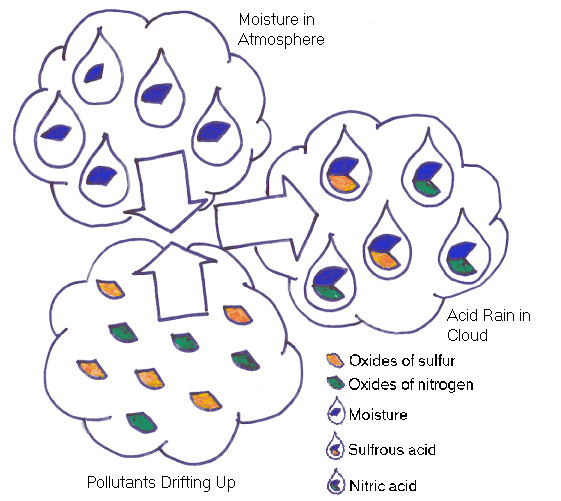
The sulfur and nitrogen compounds that contribute to acid rain primarily come from man-made sources, such as industries and utilities. Emissions also come from automobiles and other forms of transportation and industrial processes, such as smelting. Acid rain can harm forests and crops, damage bodies of water, and contribute to the damage of statues and buildings. Researchers are considering the possible effects of acid rain on human heath. These acidic pollutants can be deposited through rain, snow, fog, dew, or sleet. Large quantities can also be deposited in a dry form through dust. Pollutants that contribute to acid rain may be carried hundreds of miles before being deposited on the earth. Because of this, it is sometimes difficult to determine the specific sources of these acid rain pollutants.


