mold and mildew growth

Experiment 13 Hard versus Soft: How Do You Like Your Water? |
Introduction/Background
| The presence of Ca2+ and Mg2+ in water results in "hard" water. These ions in water react with heat, metallic plumbing, and soaps to result in several unwanted consequences. One of the most effective and efficient ways of "softening" water is by using a technique called ion exchange process. The ion exchange water softening process can remove nearly all calcium and magnesium ions from your water. Softeners may also remove as much as 5-10 ppm (parts per million; ppm is equal to milligrams per liter, or mg/L) of iron and manganese (ions that cause rust). However, water softening does not remove bacteria, hydrogen sulfide, silt or sand, lead, nitrate, pesticides, and many other organic and inorganic compounds. Ion exchange involves the removal of the hard ions (calcium and magnesium) and replacing them with softer ions (such as sodium). |  mold and mildew growth |
The softener contains a porous resin, usually polystyrene beads with sulfur groups attached that are supersaturated with sodium to cover the bead surfaces. As water passes through, calcium and magnesium ions attach to the resin beads and the loosely held sodium ion is released from the resin into the water. After softening a large quantity of hard water the beads become saturated with calcium and magnesium ions. When this occurs, the exchange resin must be regenerated, or recharged. This is done by flushing the beads with a salt solution. The sodium ions in the salt solution are exchanged with the calcium and magnesium ions on the resin and excess calcium and magnesium ions are flushed out with waste water.
| R-COO-Na + Ca2+ = R-COO-Ca + 2 Na+ **Where R = ion-exchange resin. The ion-exchange resin is used in water softeners where less soluble metals such as calcium and magnesium ions are exchanged for the more water-soluble sodium ions. |
Key Concepts
This laboratory demonstrates that natural water doesn't simply consist of solely H2O. One reason for this is that as water moves along or beneath the Earth's surface, it dissolves minerals from the rocks and soil. Minerals (or salts) are ionic, meaning they carry a positive or negative charge. Water containing calcium, magnesium, and/or iron ions is called hard water. These ions are less soluble in water and bind with the negative ions in soap to form scum. This scum clings all too well to clothes and sticks to the walls of your bathroom (bathtub ring). Sodium ions are much more soluble in water, and thus is the reason it is used in water softening procedures.
Note: The most common type of soap has as its major ingredient sodium stearate, NaC18H35O2, the sodium salt of an organic acid. The calcium and magnesium salts of this acid are insoluble in water; the reaction taking place when soap is used in hard water may be expressed as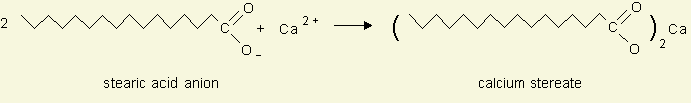 Soap scum: http://www.cci.unl.edu/Chemistry/LABS/LABSGIFs/L12_2.GIF |
Glossary
Related Materials
For more about ion exchange process in water treatment:
Fundamentals of Water Softening (http://www.culliganmiami.com/pf7.html)